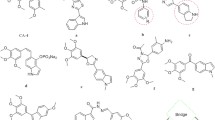
Abstract
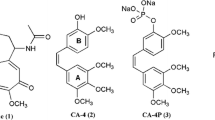
We reported the design, synthesis, and biological evaluation of 9 novel benzofuran analogues of CA-4 and their preliminary structure-activity relationship with inhibitory activity on microtubules and the phosphoinositide 3-kinase (PI3K). Various spectroscopic techniques like 1H NMR, 13C NMR, and HRMS were used for characterization of all synthesized analogs. Among these derivatives, most compounds inhibited the growth of breast tumor cell lines, especially one compound showed prominently inhibitory activity in MCF-7 and MDA-MB-231 cells with IC50 value as 3.88 and 5.78 μmol/dm3. Furthermore, we found that the same compound reduced the PI3K expression levels, conversely elevated the β-tubulin expression levels in enzyme-linked immunosorbent assay. The binding interactions between synthesized analogs and colchicine active site of tubulin protein and the PI3Kα protein were confirmed through molecular docking study. These preliminary results showed that this compound has the potency for further study as a dual-target anti-tumor inhibitor.
Graphical abstract






Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
References
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL (2022) CA - Cancer J Clin 72:524
Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC (2014) Breast 23:128
Chen R, Yuan D, Ma JJ (2022) Future Med Chem 14:97
Bhogal T, Cameron D, Palmieri C (2022) Breast 63:85
Cavalcanti I, Cabral A, Santos R (2017) Ars Pharm 58:171
Lakhani N, Orloff M, Fu S, Liu Y, Wang Y, Zhou H, Lin K, Liu F, Yan S, Patnaik A (2020) J Immunother Cancer 8:A322
Shah AS, Surnar B, Kolishetti N, Dhar S (2022) Annu Rev Mater Res 3:14
Alpízar-Pedraza D, Veulens ADLN, Araujo EC, Piloto-Ferrer J, Sánchez-Lamar A (2022) J Mol Struct 1259:132723
Pettit GR, Minardi MD, Hogan F, Price PM (2010) J Nat Prod 73:399
Young SL, Chaplin DJ (2004) Expert Opin Inv Drug 13:1171
Karatoprak GS, Akkol EK, Genç Y, Bardakci H, Yücel Ç, Sobarzo-Sánchez E (2020) Molecules 25:2560
Li XN, Tian HQ, Zhang WG (2017) J Med Chem 27:388
Schmitt F, Gosch LC, Dittmer A, Rothemund M, Mueller T, Schobert R, Biersack B, Volkamer A, Höpfner M (2019) Nat Rev Neurosci 20:383
Wu M, Sun Q, Yang C, Chen D, Ding J, Chen Y, Lin L, Xie Y (2007) Bioorg Med Chem Lett 17:869
Kamal A, Reddy NVS, Nayak VL, Reddy VS, Prasad B, Nimbarte VD, Srinivasulu V, Vishnuvardhan MVPS, Reddy CS (2014) ChemMedChem 9:117
Lee J, Bae S, Lee SH, Choi H, Kim YH, Kim SJ, Park GT, Moon SK, Kim DH, Lee S, Ahn SK, Choi NS, Lee KJ (2010) Bioorg Med Chem Lett 20:6327
Romagnoli R, Oliva P, Salvador MK, Camacho ME, Padroni C, Brancale A, Ferla S, Hamel E, Ronca R, Grillo E, Bortolozzi R, Rruga F, Mariotto E, Viola G (2019) Eur J Med Chem 181:111577
Yang J, Yu Y, Li Y, Yan W, Ye H, Niu L, Tang M, Wang Z, Yang Z, Pei H, Wei H, Zhao M, Wen J, Yang L, Ouyang L, Wei Y, Chen Q, Li W, Chen L (2021) Sci Adv 7:eabg4168
Fan Y, Luo Y, Ma C (2017) Monatsh Chem 148:1823
Lavanya A, Narasimhan K, Padmini V (2020) Mini-Rev Org Chem 17:224
Miao YH, Hu YH, Yang J, Liu T, Sun J, Wang XJ (2019) RSC Adv 9:27510
Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G (1996) Mol Cell Biol 16:1722
Venkatesan AM, Dehnhardt CM, Chen Z, Santos ED, Santos OD, Bursavich M, Gilbert AM, Ellingboe JW, Ayral-Kaloustian S, Khafizova G, Brooijmans N, Mallon R, Hollander I, Feldberg L, Lucas J, Yu K, Gibbons J, Abraham R, Mansour TS (2010) Bioorg Med Chem Lett 20:653
Huang XF, Chen JZ (2009) Obes Rev 10:610
Riquelme I, Tapia O, Espinoza JA, Leal P, Buchegger K, Sandoval A, Bizama C, Araya JC, Peek RM, Roa JC (2016) Pathol Oncol Res 22:797
Zhao Z, Zhang M, Li C, Wang X (2020) J Med Chem 8:51
Bohnacker T, Prota AE, Beaufils F, Burke JE, Melone A, Inglis AJ, Rageot D, Sele AM, Cmiljanovic V, Cmiljanovic N, Bargsten K, Aher A, Akhmanova A, Díaz JF, Fabbro D, Zvelebil M, Williams RL, Steinmetz MO, Wymann MP (2017) Nat Commun 8:14683
Ducki S, Rennison D, Woo M, Kendall A, Chabert JFD, Mcgown AT, Lawrence NJ (2009) Bioorg Med Chem 17:7698
Massue J, Frath D, Retailleau P, Ulrich G, Ziessel R (2013) Chem Eur J 19:5375
Acknowledgements
The study was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (No.2021D01F40) and Xinjiang Second Medical College Research Fund General Project (No.MS202301). We thank it for the financial support given to the research projects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Li, J. & Ma, T. Design, synthesis, and anti-breast tumor activity of novel combretastatin A-4 analogues. Monatsh Chem 154, 1285–1294 (2023). https://doi.org/10.1007/s00706-023-03127-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03127-7