Abstract
Physicochemical properties of pharmacological interest were determined for ten 6H-pyrimido[2,1-a]isoindoles. The compounds studied were found to be weak bases with a pKa ranging from 2.38 to 3.46. Furthermore, the association constants of the studied compounds with cyclodextrins were examined. The formation of complexes was observed with 1:1 stoichiometry, γ-cyclodextrin was found to be the best complexing. Finally, the electrochemical oxidation of 6H-pyrimido[2,1-a]-isoindoles in 0.1 mol dm−3 sodium perchlorate in acetonitrile was studied as a model of their possible metabolic degradation. It was found to be a one-electron process, and the values of the half-wave potentials are in the range of 1.34–1.62 V (vs. Ag/AgNO3/NaClO4). The electrooxidation products of three selected compounds were prepared by preparative electrolysis and subsequently identified by mass spectrometry. From the data obtained, it is evident that the electrochemical oxidation of the 6H-pyrimido[2,1-a]isoindoles begins with the formation of N-oxides, followed by dimerization of the molecule or, contrary to this, by oxidative cleavage of the pyrimidine ring. From a metabolic point of view, N-oxidation is the corresponding process to this pathway.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Nitrogen-containing heterocyclic compounds are the basis of a number of pharmaceutically active compounds [1]. Among the promising pharmacologically active nitrogen-containing heterocyclic compounds are derivatives of pyrimidoisoindole, first synthesized in 1961 [2]. An example of a compound from this group that has found clinical use is the antidepressant and anorectic drug ciclazindol [3]. Structural analogs of this compound have been shown to have hypoglycaemic effects [4]. To broaden the spectrum of effects of the known pyrimidoindoles, other derivatives are being synthesized; of which pyrimidoisoindoles have an important place. Syntheses of pyrimidoisoindoles are mainly dealt with by Babichev and Kovtunenko and co-workers [5]. Diuretic [6], anorectic [7], anti-HIV [8], and Leishmanicidal [9] activity have already been demonstrated for pyrimidoisoindoles. Nevertheless, research on these promising compounds is still in its early stages.
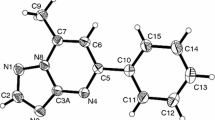
In this work, we focus on ten derivatives of 6H-pyrimido[2,1-a]isoindole (Fig. 1), which have shown promising antifungal [10] and anti-malarial [11] effects. The compounds studied can be divided into two groups according to the position of the ketone group: (i) 6H-pyrimido[2,1-a]isoindol-4-one derivatives (compounds 1, 3, 5, 7, and 9) and (ii) 6H-pyrimido[2,1-a]isoindol-2-one derivatives (compounds 2, 4, 6, 8, and 10). Moreover, compounds 5–10 are annelated derivatives, where the addition of an additional acceptor ring moiety to the electron-donating isoindole ring strongly influences the structures and reactivities of these tricyclic systems, and unusual chemical properties can be expected. For the separation of these compounds, we have previously proposed the non-aqueous capillary electrophoretic method [12].
In the first part of the work we determined the dissociation constants of the 6H-pyrimido[2,1-a]isoindoles studied as one of the basic physicochemical parameters. Dissociation significantly influences the fate of the drug in the organism, especially its permeation through lipid membranes, and thus significantly affects the resulting pharmacological effect.
The second part of the work is devoted to the study of the interaction of the 6H-pyrimido[2,1-a]isoindoles with cyclodextrins to form inclusion complexes based on non-covalent interactions. Due to their hydrophobic cavity, cyclodextrins can serve as efficient transporters of drugs, especially those that are poorly soluble in water, and, in addition, they also increase the stability of drugs in pharmaceutical preparations [13,14,15].
In the third part, we investigate the electrochemical behavior of the 6H-pyrimido[2,1-a]isoindoles studied, and, in particular, their electrochemical oxidation as the simplest model of possible biotransformation of these compounds in a living organism. Electrochemical methods are highly useful tools for pharmacology [16] and the electrochemical approach has been proven for the biomimetic modeling of oxidative drug metabolism [17,18,19,20]. It has been shown that electrochemical oxidation of a drug can produce a compound that is identical to the metabolite of the drug in question [21,22,23].
Results and discussion
Acid–base properties of 6H-pyrimido[2,1-a]isoindoles
Due to the lower solubility of the 6H-pyrimido[2,1-a]isoindoles in water, the dissociation constants were determined by UV–Vis spectrometry according to Blume et al. [24] in a mixed water–methanol environment (the concentration of methanol in the resulting solutions was a maximum of 5%). The pH values of the solutions were ensured using acetate or phosphate buffer. Figure 2 shows an example of the changes in the spectrum of compound 8 as a function of the pH of the medium. The determined values of the dissociation constants are summarized in Table 1.
The determined values of the dissociation constants range from 2.38 to 3.46; of course, 6H-pyrimido[2,1-a]isoindoles are weak organic bases, like most nitrogen heterocycles. The dissociation constant of the basic pyrimidine is pKa = 1.3 [25] and shifts to higher values due to the presence of a ketone group; for example, 3H-pyrimidin-4-one has pKa,1 = 1.64 and pKa,2 = 8.6 [26]. For the 6H-pyrimido[2,1-a]isoindoles studied, the substituent at position 2 has an increasing effect on the value of the dissociation constant, while the same substituent at position 4 leads to a decrease in the value of the dissociation constant. The magnitude of the effect is naturally determined by the type of substituent. However, when the substituent is an annelated ring, the values of the dissociation constant are higher for the annelated ring on the [c] side than for the ring which is annelated on the [b] side.
Complexation properties of 6H-pyrimido[2,1-a]isoindoles with cyclodextrines
The determination of the association constants of the 6H-pyrimido[2,1-a]isoindoles studied was carried out using UV–Vis spectrometry by monitoring the change in absorbance at the absorption maximum wavelength as a function of different concentrations of cyclodextrin. Evaluation of stoichiometry and association constants was performed using the Benesi-Hildebrandt method [27]. To ensure that only the non-protonated form of 6H-pyrimido[2,1-a]isoindole is present in the solution, an environment with constant pH = 6.8, which is also close to physiological values, was chosen based on the observed values of the dissociation constants. All the 6H-pyrimido[2,1-a]isoindoles studied formed inclusion complexes with γ-cyclodextrin, which has the largest volume of internal cavity; for α- and β-cyclodextrin, some derivatives did not form complexes. The stoichiometry of 6H-pyrimido[2,1-a]isoindole: cyclodextrin was found to be 1:1 in all cases. The values of the association constants obtained are given in Table 2.
From the measured values of the association constants it is evident that the value of the association constant depends both on the type of cyclodextrin used and the spatial structure of 6H-pyrimido[2,1-a]isoindole. The distribution of the negative charge localized on the oxygen atom – which is influenced by the position of the ketone group and the substituent, or the annelated ring—plays a major role. The association constant is further affected by the planarity of the molecule; for example, compounds 7 and 8 are the least planar, with the annelated ring in the twist conformation. In addition, the cavity of cyclodextrin may be encapsulated by the ring of the isoindole part of the molecule or, conversely, by a substituent on the pyrimidine ring. This may explain the large differences in the values of association constants of individual 6H-pyrimido[2,1-a]isoindoles both with each other or for the same 6H-pyrimido[2,1-a]isoindole and different cyclodextrins. For example, compounds 2 and 4 differ in the type of substituent at position 4. Compound 2 (with a methoxy group at position 4) has a greater association constant with γ-cyclodextrin and the smallest with β-cyclodextrin. On the other hand, compound 4 (with a phenyl at position 4) has the largest interaction with β-cyclodextrin and the smallest with α-cyclodextrin. Compound 7, which interacts slightly with γ-cyclodextrin, showed only a slight increase in the values of the association constants when the presence of α-cyclodextrin is replaced by β-cyclodextrin and vice versa.
Voltammetric behavior of 6H-pyrimido[2,1-a]-isoindoles
Using DC voltammetry on a rotating disk electrode, it was found that all the 6H-pyrimido[2,1-a]isoindoles studied were oxidized in a single step in 0.1 mol dm−3 sodium perchlorate in anhydrous acetonitrile. Basic electrochemical parameters are given in Table 3. The values of the number of electrons exchanged were determined by potentiostatic coulometry. The diffusion coefficients of the 6H-pyrimido[2,1-a]-isoindoles studied were calculated from the dependence Ilim = f(ω1/2) by trim Levitsch’s equation [28].
From these results it is evident that the presence of a terminal non-aromatic ring in the compound significantly reduces the half-wave potential and, consequently, the energetic demand of oxidation (compounds 5, 6, 7, and 8). For derivatives with a ketone group at the 2 position (compound 2, 4, 6, 8, and 10), the concentration plots were linear to lower concentrations than for 6H-pyrimido[2,1-a]-isoindol-4-ones. It is probably related to the structure of the compound; adsorption waves were formed at higher concentrations for 6H-pyrimido[2,1-a]isoindol-2-ones. The measured values of the exchanged electrons ranged from 0.97 to 1.35; therefore, it is evident that the oxidation of the 6H-pyrimido[2,1-a]isoindoles studied is one-electron process. From the observed values of diffusion coefficients, it is evident that the 6H-pyrimido[2,1-a]isoindol-2-ones have significantly lower values of diffusion coefficients than the 6H-pyrimido[2,1-a]isoindol-4-ones. Again, this is related to the structure of the compound. The presence of the terminal aromatic ring in the structure (compound 9 and 10) resulted in a decrease in the difference in the diffusion coefficient value of compounds with different position of the ketone group.
The reversibility of the electrochemical oxidation of the 6H-pyrimido[2,1-a]isoindoles studied was monitored by cyclic voltammetry. In the whole range of electrode polarization rates of 0.01–15 V s−1 only the corresponding anodic peaks were observed for all compounds studied; thus, the electrochemical oxidation of 6H-pyrimido[2,1-a]isoindoles is a totally irreversible process (Fig. 3).
Electrooxidation of 6H-pyrimido[2,1-a]isoindoles as a model of metabolic degradation.
Using preparative electrolysis [22, 23], the electrooxidation products of compounds 1, 2, and 5 were prepared and analyzed. An amount of 10 mg of the compound was oxidized in 40 cm3 of 0.1 mol dm−3 sodium perchlorate in anhydrous acetonitrile at a potential corresponding to the limiting diffusion current, that is, for compound 1 at 1.90 V, for compound 2 at 1.82 V, and for compound 5 at 1.60 V. The electrolysis time was 2 h.
A slow precipitation of yellow precipitate was observed in the free-standing solution after electrolysis of compound 5, therefore, the solutions were stored immediately after electrolysis by freezing in dry ice. Using mass spectrometry, 1H-isoindole-1,3 (2H)-dione (Fig. 4, compound 11) was found as a product of the electrochemical oxidation of compounds 1 and 2. Electrolysis of compound 5 gave two products, which are N-oxides (Fig. 4, compound 12 and 13). The yellow precipitate was identified as dione (Fig. 4, compound 14).
From the results obtained, it is possible to hypothesize the following probable course of electrochemical oxidation of the 6H-pyrimido[2,1-a]isoindoles studied. With any compound, electrochemical oxidation begins with the elimination of one electron from the nitrogen atom at position 1, and the resulting radical has only a very short lifetime. This radical undergoes either a dimerization reaction (which is manifested by the formation of a yellow precipitate from the solution after electrolysis), a very common reaction pathway in the oxidation of nitrogen heterocycles, or by a reaction with traces of water present in the reaction medium to form N-oxides. In the case of derivatives without a side ring (compounds 1–4), oxidative cleavage of the pyrimidine ring occurs to form dions. The presence of the terminal ring is likely to have a stabilizing effect on the resulting N-oxide and prevent oxidative cleavage of the pyrimidine ring.
This proposed mechanism is in agreement with known data on the electrochemical, chemical, and/or biological oxidation of pyrimidine derivatives. In the literature, the corresponding N-oxides have been described as products of the electrochemical oxidation of indoline [29] or isoindole [30]. Jennings et al. [31] reported, that the electrochemical oxidation of a wide range of 5-substituted indole monomers leads to the formation of various dimers, trimers and polymers. The formation of N-oxides is also typical for the chemical oxidation of various heterocyclic nitrogen-containing compounds [32, 33]. The biological oxidation of nitrogen heterocyclic compounds is also characterized by the so-called N-oxidation catalyzed by the cell microsomal system [34]. In phase II of biotransformation, N-oxides are further conjugated to N-glucuronides [35], alternatively O-glucuronides may be formed in the case of the resulting diones [36]. Oxidative cleavage of the pyrimidine ring has also been observed for the predecessor of the 6H-pyrimido[2,1-a]-isoindoles studied, the clinically used ciclazindol [37]. Similarly, cleavage of the indole ring was observed for proterguride after prior N-deethylation and N-oxidation [38].
Conclusions
In the first part of the work the dissociation constants of the 6H-pyrimido[2,1-a]isoindoles studied, which are weak organic bases, were determined. It was found that the value of the dissociation constant depends mainly on the position of the ketone group and also on the structure of the substituent. In the second part of the work, the associations constant values with cyclodextrins, with which 6H-pyrimido[2,1-a]-isoindoles form complexes with a stoichiometry of 1:1, were determined. Also, in this case, the value of the association constant is influenced by the structure of the particular compound. Furthermore, the basic electrochemical parameters for the anodic oxidation of 6H-pyrimido[2,1-a]isoindoles in non-aqueous media were studied. Electrochemical oxidation was also used successfully as a simple model for phase I biotransformation. As 6H-pyrimido[2,1-a]isoindoles have not yet been examined in vivo, the results obtained represent beneficial clues to their possible metabolic fate.
Experimental
Chemicals
The 6H-pyrimido[2,1-a]isoindoles studied were prepared according to Ishchenko et al. [39]. The identity and purity of the substances were confirmed by elemental analysis, melting point measurement, thin layer chromatography, infrared spectrometry, and NMR. Stock solutions of the compounds studied to determine the dissociation and association constants were prepared with a concentration of 5 × 10−3 mol dm−3 in methanol. All other chemical used were of p.a. or higher quality: acetic acid 99% (Lach-Ner, Czech Republic), acetonitrile (Aigma-Aldrich), α-cyclodextrin (Sigma-Aldrich), β-cyclodextrin (Sigma-Aldrich), γ-cyclodextrin (Sigma-Aldrich), hydrochloric acid 35% (Lach-Ner, Czech Republic), methanol (Merck), phosphoric acid 85% (Lachema, Czech Republic), potassium dihydrogenphosphate (Lach-Ner, Czech Republic), silver nitrate (Lach-Ner, Czech Republic), sodium dihydrogen phosphate (Lach-Ner, Czech Republic), sodium hydroxide (Lach-Ner, Czech Republic), sodium perchlorate (Sigma-Aldrich).
Instrumentation
An Agilent 8453 UV–visible Spectroscopy System spectrometer was used to measure absorption spectra. The measurements were performed in a quartz cuvette with 1.00-cm absorption layer. The pH measurements were performed on a Jenway 3305 pH meter with a combined glass electrode. Three-point glass electrode calibration at pH = 4.01 ± 0.01, 7.01 ± 0.01, and 10.01 ± 0.01 (HANNA Instruments, USA) was performed. Because the presence of inorganic salts (used to prepare buffers) can promote aggregation with cyclodextrins, the pH of the solution was adjusted by adding an appropriate volume of 0.1 mol dm−3 HCl or 0.1 mol dm−3 NaOH when measuring the association constants [14]. The DC and cyclic coltammetry measurements were performed on an Eko-Tribo Polarograph (Polaro-Sensors, Czech Republic) [22, 23]. A platinum disk electrode with an active surface area of 0.111 cm2 was used. The reference electrode was a silver plate immersed in a solution of 0.01 mol dm−3 AgNO3 in 1.00 mol dm−3 NaClO4 in acetonitrile and separated from measured solution by a salt bridge filled with 0.5 mol dm−3 NaClO4 in acetonitrile. The platinum rod served as a counter electrode. The method of potentiostatic coulometry is described in our previous work [40]. Preparative electrolysis details are published in our previous works [22, 23]. The products prepared by preparative electrolysis were separated using a Varian 3400 gas chromatograph connected with a Finnigan MAT INCOS 50 mass spectrometer. Separation was carried out on a DB 5 column (30 m × 0.25 mm i.d.; film thickness 0.012 μm) at 250 °C. Electron impact ionization was used.
Data availability
The experimental data that support the findings of this study are available from the corresponding author, K.N., upon reasonable request.
References
Heravi MM, Zadsirjan V (2020) RSC Adv 10:44247
Bortnick NM, Fegley MF (1961) Condensed heterocyclic nitriles. Chem Abstr 56:18335
Ghose K, Rama Rao VA, Bailey J, Coppen A (1978) Psychopharmacology 57:109
Cliffe IA, Lien EL, Mansell HL, Steiner KE, Todd RS, White AC, Black RM (1992) J Med Chem 35:1169
Pokholenko AA, Voitenko ZV, Kovtunenko VA (2004) Russ Chem Rev 73:771
Mayor C, Wentrup C (1975) J Am Chem Soc 97:7467
Aeberli P, Eden P, Gogerty JH, Houlihan WJ, Penberthy C (1975) J Med Chem 18:177
Bedoya LM, del Olmo E, Sancho R, Barboza B, Beltrán M, García-Cadenas AE, Sánchez-Palomino S, López-Pérez JL, Muñoz E, San Feliciano A, Alcamí J (2006) Bioorg Med Chem Lett 16:4075
del Olmo E, Armas MG, López-Pérez JL, Muñoz V, Deharo E, San Feliciano A (2001) Bioorg Med Chem Lett 11:2123
Nesměrák K, Pelouchová H, Všetečka V, Němec I, Gabriel J (1998) Fol Microbiol 43:39
Chen X, Xia F, Zhao Y, Ma J, Ma Y, Zhang D, Yang L, Sun P (2020) Chin J Chem 38:1239
Pumera M, Horká V, Nesměrák K (2002) J Sep Sci 25:443
Jacob S, Nair AB (2018) Drug Dev Res 79:201
Němcová I, Nesměrák K, Kafková B, Sejbal J (2006) Collect Czech Chem Commun 71:179
Raut SY, Manne ASN, Kalthur G, Jain S, Mutalik S (2019) Curr Pharm Des 25:444
Nesměrák K (2020) Mini-Rev Med Chem 20:1341
Lohmann W, Karst U (2008) Anal Bioanal Chem 391:79
Álvarez-Lueje A, Dragnic SB (2010) Comb Chem High Throughput Screen 13:712
Portychová L, Schug KA (2017) Anal Chim Acta 993:1
Faber H, Vogel M, Karst U (2014) Anal Chim Acta 834:9
Jurva U, Weidolf L (2015) TrAC Trends Anal Chem 70:92
Nesměrák K, Doležal R, Hudská V, Bártl J, Štícha M, Waisser K (2010) Electroanalysis 22:2117
Nesmerak K, Nemec I, Sticha M, Waisser K, Palat K (2005) Electrochim Acta 50:1431
Blume R, Lachmann H, Mauser H, Schneider F (1974) Z Naturforsch B J Chem Sci 29:500
Albert A, Goldacre R, Phillips J (1948). J Chem Soc. https://doi.org/10.1039/jr9480002240
Brown DJ, Short LN (1953). J Chem Soc. https://doi.org/10.1039/jr9530000331
Connors KA (1987) Binding constants: the measurement of molecular complex stability. Wiley, New York
Opekar F, Beran P (1976) J Electroanal Chem Interfacial Electrochem 69:1
Alberti A, Andruzzi R, Greci L, Stipa P, Marrosu G, Trazza A, Poloni M (1988) Tetrahedron 44:1503
Lindgren A, Eklund G, Turek D, Malmquist J, Swahn BM, Holenz J, von Berg S, Karlström S, Bueters T (2013) Drug Metab Dispos 41:1134
Jennings P, Jones AC, Mount AR, Thomson AD (1997) J Chem Soc Faraday Trans 93:3791
Kobayashi Y, Kumadaki I, Sato H, Sekine Y, Hara T (1974) Chem Pharm Bull (Tokyo) 22:2097
Tang Y, Li K, Chinnam AK, Staples RJ, Shreeve JM (2021) Dalton Trans 50:2143
Altuntas TG, Gorrod JW (1996) Xenobiotica 26:9
Mattiuz E, Franklin R, Gillespie T, Murphy A, Bernstein J, Chiu A, Hotten T, Kassahun K (1997) Drug Metab Dispos 25:573
Stachulski AV, Jenkins GN (1998) Nat Prod Rep 15:173
Swaisland AJ, Franklin RA, White AC (1979) Br J Clin Pharmacol 7:120
Krause W, Düsterberg B, Jakobs U, Hoyer GA (1993) Drug Metab Dispos 21:203
Ishchenko VV, Kovtunenko VA, Tyltin AK, Trachevskii VV, Vshetechka V, Babichev FS (1990) Ukr Khim Zh (Russ Ed) 56:517
Nesměrák K, Němec I, Štícha M, Gabriel J, Mirceski V (1999) Collect Czech Chem Commun 64:1100
Acknowledgements
The financial support by the project Cooperation of Charles University is gratefully acknowledged.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nesměrák, K., Němcová, I. 6H-Pyrimido[2,1-a]isoindoles: acid–base and complexation properties and electrooxidation model of metabolic degradation. Monatsh Chem 154, 1035–1041 (2023). https://doi.org/10.1007/s00706-023-03075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03075-2