Abstract
Trimetaphosphimates decompose not only upon heating but also in water. Hydrolysis of trimetaphosphimic acid leads to the formation of diimidotriphosphoric acid by ring opening. Single-crystal structure determination using synchrotron radiation revealed the oxonium salt of the latter acid with double stacks of rod-shaped anions along [001]. Anharmonic displacement parameters were used to model slight disorder of part of the anions. These are interconnected by hydrogen bonds within and between the stacks. Powder X-ray diffraction confirmed phase purity. At temperatures above 120 °C, diimidotriphosphoric acid decomposes into unknown crystalline compounds. It thus seems a promising precursor for the synthesis of oxonitridophosphates at moderate temperatures.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Not much is known about imidophosphoric acids and their salts. In these compounds, NH groups interconnect P atoms in condensed phosphates, formally replacing bridging O atoms. Such compounds have been described as catalysts for various reactions, e.g., Oxa-Pictet–Spengler or Mannich reactions or N–O acetalizations [1, 2]. Imidophosphoric acids or their salts can be synthesized in different ways. Ammonolysis of cyclophosphates and subsequent recyclization and rearrangement reactions have been described as one feasible approach [3]. Comparable products are obtained by the reaction of hydrochloric acid and phosphoric triamide [4] or the solvolysis of linear chlorophosphazenes with formic acid and subsequent treatment with ammonia [5]. In a similar way, the hydrolysis of trimetaphosphimates may open their P3N3 ring, forming amidodiimidotriphosphates as described for the reaction of sodium trimetaphosphimate with water [6,7,8]. Further hydrolysis leads to the formation of various polyphosphates and ammonia or ammonium ions. Similar products can be expected for hydrolysis of other trimetaphosphimates or the acid itself. The latter crystallizes as an oxonium salt [9, 10]. Oxonium ions have, in fact, been reported in the crystal structures of several phosphates, such as oxonium iron(III) orthophosphate hydrate [11], oxonium uranyl phosphate trihydrate [12], and oxonium antimony phosphate hydrate [13].
Trimetaphosphimates are possible precursors for oxonitridophosphates, imidonitridophosphates, or oxoimidophosphates as they decompose upon heating. NaPO2NH, for instance, has been obtained from sodium trimetaphosphimate [14]. Volatile compounds like H2O or NH3 formed during condensation are the driving force of these reactions. Similar precursor chemistry may be envisaged with rod-shaped imidooligophosphates. This is intriguing as many oxonitridophosphates are comparable to silicates because PON and SiO2 are isoelectronic. Both classes of compounds form tetrahedral frameworks and have similar but not the same properties. Thus, they complement each other and allow advanced tuning of properties, e.g., in luminescence materials [15, 16].
Whereas numerous crystal structures of cyclic trimetaphosphimates are known [17], rod-shaped imidooligophosphates have not yet been characterized by X-ray diffraction (an imidodiphosphate is published in Ref. [18]). Herein we present the crystal structure of oxonium diimidotriphosphate hydrate 1, (H3O)(PO3H2–NH–PO2–NH–PO3H2) × 0.5H2O, an intermediate of the hydrolysis of trimetaphosphimic acid.
Results and discussion
The initial synthesis aimed at trimetaphosphimic acid by the reaction of its potassium salt with perchloric acid to remove potassium. As mentioned in the introduction, trimetaphosphimates undergo slow hydrolysis in presence of water, resulting in ammonia or ammonium ions and (imido-)phosphates. Ring opening should lead to amidodiimidotriphosphate, which further hydrolyzes to a diimidotriphosphate according to Eq. (1). Further hydrolysis is expected, but in the present case, it is most likely prevented by low water concentration in the acetone-rich solvent mixture. Instead, the oxonium salt hydrate (H3O)(PO3H2–NH–PO2–NH–PO3H2) × 0.5H2O (1) crystallized from the filtrate after a batch on trimetaphosphimic acid had been removed by filtration. Comparison of a measured powder X-ray diffraction (PXRD) pattern with a calculated one (Fig. S1 in the Supporting Information, henceforth SI) based on the result of single-crystal X-ray diffraction (SCXRD) confirms that 1 is the only product of the secondary crystallization. Slight intensity misfits in Fig. S1 are probably due to preferred orientation and the fact that anharmonic displacement parameters used for SCXRD structure refinement were not taken into account in the simulated PXRD pattern.
The crystal structure was determined by SCXRD using synchrotron data acquired at beamline P24 (DESY, Hamburg) at room temperature (RT) and slightly below the decomposition temperature (120 °C). 1 crystallizes in space group C2/c. The structure was initially solved using direct methods, completed by difference Fourier analysis and refined by least-squares methods. R values corroborate the assignment of O and N atoms, which was thoroughly checked to exclude terminal NH2 instead of OH. Replacing one O atom of the terminal phosphate groups by N increases R1 (for observed reflections) from 0.0374 to ~ 0.040 with slight variation depending on the O position tested. Moreover, the bond lengths of ~ 1.48 and ~ 1.54 Å correspond well to P = O and P–OH bond lengths, respectively, observed for trimetaphosphimate ions [10]. P–NH bond lengths of ~ 1.65 Å also match corresponding ones in these related ions very well. All H atoms could be located as significant difference electron densities, including three positions at distances expected for O–H bonds near one of the “solvent molecules”, which thus turned out to be an oxonium ion (Fig. S2 in SI). The presence of an ammonium ion is unlikely as changing atom type to N does not decrease R values (but increases them by ~ 2% of their values) and as there is no fourth difference density maximum, However, we cannot rule out that a small percentage of oxonium might be substituted by ammonium. In addition, difference Fourier maps around both O atoms connected to the P atom in the middle of the rod-shaped molecule did not show any residual electron density that would correspond to a H atom. In addition, the respective P–O distances are shorter than expected for P–OH. Thus, deprotonation takes place in the middle of the acid molecule. Therefore, we conclude that 1 crystallizes as an oxonium salt comparable to trimetaphosphimic acid in the solid state [9, 10]. Although all H atoms can be refined on positions indicated by difference Fourier synthesis, soft restraints for bond lengths (0.85 for OH bonds and 0.89 for NH bonds) and bond angles (tetrahedral) were applied to ensure typical molecular geometry. As data quality decreases with temperature, more restraints for H atoms were used for the high-temperature data set, keeping the H bonding scheme similar to that in the RT structure. One of the PO3H2 groups shows significant disorder, which cannot be well resolved by split-atom models. Therefore, the corresponding O atom sites were described with anharmonic displacement parameters (see below). Refinement results are listed in Table 1, atomic parameters in Tables S1–S6 in the SI.
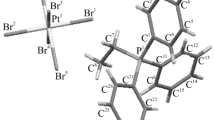
The rod-shaped anions of 1 form double stacks along [001] (Fig. 1 and Fig. S3 in the SI). Each anion forms four H bonds to adjacent molecules of the same stack and additional four H bonds to one anion of the neighboring stack. Two further H bonds connect each anion to another anion of a neighboring double stack. Seven of the ten H atoms per asymmetric unit form rather linear H bonds with angles > 158° (Table 2 and Fig. 2), all of which correspond to medium strong H bonds with respect to H─acceptor distances as well as donor─H─acceptor angles [20]. However, the almost linear H bonds for HO2 and HO3 of the ordered phosphate group are significantly stronger than the others and most likely prevent disorder. Oxonium ions and water molecules are located between the stacks. One H atom (HO9a) of the oxonium ion forms a bifurcated H bond with rather weak contacts to O atoms of different anions. Although the other H atoms of the oxonium ion also have two possible acceptors at distances < 3 Å, the corresponding H bonds are not classified as bifurcated as one of the O–H distances is shorter. However, they deviate strongly from linearity.
The O atoms of the disordered PO3H2 group predominantly form hydrogen bonds to two adjacent anions within a double stack, two of them to an anion of the other stack and one to an anion in the same stack. However, there are 4 and 5 possible acceptors within a range of 2.5 Å for HO6 and HO8, respectively. This enables additional less favorable but possible H bond arrangements by rotation around the P–N bond. The corresponding disorder involves large displacements of O atoms, which can neither be described by displacement ellipsoids nor by well-resolved split-atom models. Therefore, O atom positions were refined using anharmonic displacement parameters of a Gram–Charlier expansion with tensors up to the 4th rank. The joint pair distribution function (j.p.d.f.) describes the displacement of the O atoms as depicted in Fig. 3, which also shows the associated electron densities obtained from both observed and calculated structure factors. The latter match very well.
At 120 °C, displacement parameters of all atoms, of course, increase. However, this increase is much more pronounced for the O atoms of the disordered phosphate group. Their j.p.d.f. becomes smoother, indicating increasingly dynamical disorder. The corresponding electron density is more smeared out at higher temperatures due to more intense vibrations and increasing disorder.
Temperature-dependent PXRD (Fig. 4) shows that 1 decomposes at about 125 °C, followed by further reaction steps at 300 and 380 °C. Each reaction affords a different crystalline compound. None of the respective diffraction patterns could be assigned to known phases. Unfortunately, isolation of these compounds by ex-situ synthesis as well as structure solution from the high-temperature PXRD were not successful so far. Above 520 °C, the last crystalline decomposition product either melts or becomes amorphous.
Conclusion
Hydrolysis of trimetaphosphimic acid and associated ring opening led to the first observation of diimidotriphosphoric acid, which crystallizes as an oxonium salt hydrate 1 from a water/acetone mixture. The acid is deprotonated in the middle as the corresponding anion is better stabilized by resonance. The anions form double stacks along [001] and interconnected by hydrogen bonds that involve all H atoms. As there are different possible acceptors for H bonds, one -PO3H2 group exhibits a certain degree of disorder; however, one H bonding scheme clearly dominates. Upon heating above 120 °C, 1 decomposes into a series of unknown crystalline phases with increasing temperature. It may therefore be an interesting precursor for the synthesis of oxonitridophosphates at relatively low temperatures. One may speculate that the disorder enhances the reactivity of one of the terminal phosphate groups, especially at elevated temperature.
Experimental
Synthesis
Initially, the synthesis aimed at trimetaphosphimic acid from K3(PO2NH)3, both of which are described in the literature [9, 21]. 2.5 g of K3(PO2NH)3 prepared from hexachlorophosphazene and potassium acetate were dissolved in 7 cm3 of H2O and cooled to 0 °C in an ice bath. 2.5 cm3 of HClO4 (60%) was then added dropwise while continuously stirring the mixture. Precipitated KClO4 was removed by filtration and the filtrate directly passed into 100 cm3 of acetone. White crystals of trimetaphosphimic acid formed and were removed after 24 h. From the filtrate, further crystals form slowly over about 1 week at a room temperature of ca. 25–28 °C. These were isolated and 0.158 g of the title compound were obtained. Note that rather similar conditions can lead to other unknown products; especially, the water/acetone ratio seems to be decisive.
Single-crystal X-ray diffraction
SCXRD data were acquired using synchrotron radiation on a Huber kappa diffractometer at beamline P24 (DESY, Hamburg) equipped with a Pilatus CdTe detector (Dectris, Switzerland). Suitable crystals were selected using polarized light and fixed in capillaries (diameter 0.2 mm) with a glass fiber. Indexing and integration were done using the program Crysalis [22]. Scaling and semi-empirical absorption correction were carried out with SADABS [19]. Structure solution by direct methods and refinement were done with SHELXS and JANA2020, respectively [23, 24]. Diamond and VESTA were used for graphical visualization of the structure [25, 26].
Powder X-ray diffraction
PXRD patterns were acquired on a Rigaku SmartLab diffractometer equipped with a Hypix-3000 detector. Cross-beam optics with a multilayer mirror were used for focusing and monochromation of the beam (Cu–Kα1 radiation). A small amount of the powdered sample was filled into a glass capillary (diameter 0.3 mm) and sealed under Ar atmosphere. Temperature-dependent measurements between 30 and 530 °C were carried out with increments of 10 K using a HTK 1200N reaction chamber from Anton Paar.
Data availability
CCDC 2227380 and 2227385 contain supplementary crystallographic data and can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223-336-033; or by e-mail: deposit@ccdc.cam.ac.uk. Further data are available from the authors upon request.
References
Das S, Liu L, Zheng Y, Alachraf MW, Thiel W, De Kanta C, List B (2016) J Am Chem Soc 138:9432
Langdon SM (2016) Tetrahedron 72:5247
Lehman HA, Töpelmann W, Weichold R (1978) Z Chem 18:30
Riesel L, Somieski R (1975) Z Anorg Allg Chem 415:1
Riesel L, Somieski R (1975) Z Anorg Allg Chem 412:246
Thilo E, Rätz R (1949) Z Anorg Allg Chem 258:3
Wanek W (1967) Z Chem 7:1822
Wanek W, Thilo E (1969) Chem Commun 35:2712
Attig R, Mootz D (1976) Z Anorg Allg Chem 419:139
Günther D, Kalischer C, Oeckler O (2022) Z Anorg Allg Chem 648:e202200259
Bosman WP, Beureksen PT, Smits JMM, Behm H, Mintjens J, Meisel W, Fuggle JC (1986) Acta Crystallogr Sect C 42:525
Pozas-Tormo R, Moreno-Real L, Martinez-Lara M, Bruque-Gamez S (1987) Inorg Chem 26:1442
Hodson MJ, Locke W, Mitchel PCH (1995) J Mater Chem 5:159
Stock N (1998) Phosphor(V)-oxidnitride: Von molekularen und molekularionischen Vorstufen zu kondensierten Festkörpern. Ph.D. Thesis, Universität Bayreuth, Germany
Wendl S, Schmidt P, Schnick W (2021) Nitridophosphate Phosphors for solid state lighting and method of production. Patent WO2021183847 (A1). Chem Abstr 176:139489
Wendl S, Eisenburger L, Strobel P, Günther D, Wright JP, Schmidt PJ, Oeckler O, Schnick W (2020) Chem Eur J 26:7292
Correll S (2006) Dreierringe in molekularionischen Imidophosphaten und kondensierten Oxonitridophosphaten mit NPO-Zeolithstruktur. Ph. D. Thesis. Ludwig-Maximilians-Universität München, Germany
Larsen ML, Willett RD (1974) Acta Crystallogr Sect B 30:522
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) J Appl Crystallogr 48:3
Steiner T (2002) Angew Chem 114:50; Angew Chem Int Ed 41:48
Stock N, Schnick W (1997) Z Naturforsch B 52:251
CrysAlisPRO v. 171.38.41 (2015) Rigaku Oxford Diffraction Ltd., Yarnton, England
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112
Petricek V, Dusek M, Palatinus L (2014) Z Kristallogr 229:345
Brandenburg K (2020) DIAMOND 4.6.4. Crystal Impact GbR, Bonn, Germany
Momma K, Izumi F (2011) J Appl Crystallogr 44:1272
Acknowledgements
We are grateful to Prof. Holger Kohlmann for providing the SmartLab diffractometer and Simon Keilholz for conducting the PXRD measurements. We thank the Deutsches Elektronensynchrotron (DESY, Hamburg) for provided beamtime (project I-20191026) and Dr. Christopher Benndorf and Matthias Jakob for help with the measurements. The Deutsche Forschungsgemeinschaft is gratefully acknowledged for funding (grant OE530/6-1).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Günther, D., Paulmann, C. & Oeckler, O. Oxonium diimidotriphosphate from the hydrolysis of trimetaphosphimic acid. Monatsh Chem 154, 325–330 (2023). https://doi.org/10.1007/s00706-023-03053-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03053-8