Abstract
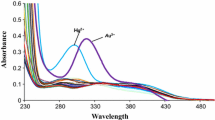
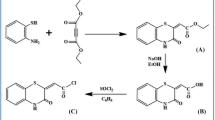
In this study, azocalix[4]arene was prepared by linking 4-methylaniline to calix[4]arene through a diazo-coupling reaction, and the targeted amide derivative was synthesized with the help of its monoester, monoacid, and monoacid chloride derivatives, respectively. The synthesis and characterization of novel trisamidetetraazocalix[4]arene [2,4,6-tris(25-aminocarbonylmethoxy-26,27,28-trihydroxy-5,11,17,23-tetrakis(4-methylphenyl)azocalix[4]arene)-1,3,5-triazine] were prepared by reacting 25-(chloroformylmethoxy)-26,27,28-trihydroxy-5,11,17,23-tetrakis(4-methylphenyl)azocalix[4]arene and melamine (2,4,6-triamino-1,3,5-triazine) to an amide formation reaction. This compound was characterized by elemental analysis, 1H NMR, IR and mass spectrometry. Picrate extraction studies were carried out from the aqueous phase to the chloroform phase with selected metal ions (K+, Sr2+, Ag+, Hg+, Hg2+, Co2+, Ni2+, Cu2+, Pb2+, Cr3+, Al3+) of the title compound. Trisamidetetraazocalix[4]arene showed extraction value in decreasing order against Hg2+ > Hg+ > Ag+ > Cr3+ > Al3+ from selected metals and showed the highest extraction value (66.2%) in Hg2+ ion.
Graphical Abstract





Similar content being viewed by others
References
Deligz H, Memon S (2011) Pak J Anal Environ Chem 12:1
Shimizu S, Shirakawa S, Suzuki T, Sasaki Y (2001) Tetrahedron 57:6169
Elçin S, Ilhan MM, Deligöz H (2013) J Incl Phenom Macrocycl Chem 77:259
Kaya A, Kutlu T, Hol A, Surucu A, Alpoguz HK (2014) Desalin Water Treat 52:3219
Sayin S, Ozbek C, Okur S, Yilmaz M (2014) J Organomet Chem 771:9
Qazi MA, Qureshi I, Memon S (2010) J Mol Struct 975:69
Lodi A, Caselli M, Casnati A, Momicchioli F, Sansone F, Vanossi D, Ponterini G (2007) J Mol Struct 846:49
Tabakci B, Yilmaz A (2014) J Mol Struct 1075:96
Ersoz M (2007) Adv Colloid Interface Sci 134:96
Karimi-Maleh H, Sanati AL, Gupta VK, Yoosefian M, Asif M, Bahari A (2014) Sens Actuators B Chem 204:647
Gassoumi B, Echabaane M, Mohamed FB, Nouar L, Madi F, Karayel A, Ghalla H, Castro ME, Melendez FJ, Özkınalı S, Rouis A, Chaabane RB (2022) Spectrochim Acta A Mol Biomol Spectrosc 264:120242
Anandababu A, Anandan S, Syed A, Marraiki N, Ashokkumar M (2021) Inorg Chim Acta 516:120133
Karaman C, Karaman O, Atar N, Yola ML (2022) Microchim Acta 189:1
Medetalibeyoğlu H, Beytur M, Manap S, Karaman C, Kardaş F, Akyıldırım O, Kotan G, Yüksek H, Atar N, Yola ML (2020) ECS J Solid State Sci Technol 9:101006
Chawla HM, Goel P, Shukla R, Black DS, Kumar N (2014) J Incl Phenom Macrocycl Chem 80:201
Deligöz H (2006) J Incl Phenom Macrocycl Chem 55:197
Yilmaz M, Deligöz H (1998) Synth React Inorg Met-Org Chem 28:851
Mokhtari B, Pourabdollah K (2012) J Incl Phenom Macrocycl Chem 73:269
Pathak RK, Hinge VK, Mondal M, Rao CP (2011) J Org Chem 76:10039
Othman AB, Mellah B, Abidi R, Kim JS, Kim Y, Vicens J (2020) J Incl Phenom Macrocycl Chem 97:187
Descalzo AB, Rurack K, Weisshoff H, Martínez-Máñez R, Marcos MD, Amorós P, Hoffmann K, Soto J (2005) J Am Chem Soc 127:184
Cho EJ, Ryu BJ, Lee YJ, Nam KC (2005) Org Lett 7:2607
Lu Y, Liao W (2016) Hydrometallurgy 165:300
Bayrakdar A, Kart HH, Elcin S, Deligoz H, Karabacak M (2015) Spectrochim Acta A Mol Biomol Spectrosc 136:607
Deligöz H (2003) Supramol Chem 15:317
Elçin S, Deligöz H (2014) J Incl Phenom Macrocycl Chem 80:337
Deligöz H, Yilmaz M (1995) Solvent Extr Ion Exch 13:19
Karakuş ÖÖ, Deligöz H (2008) J Incl Phenom Macrocycl Chem 61:289
Kanagathara N, Marchewka M, Drozd M, Renganathan N, Gunasekaran S, Anbalagan G (2013) J Mol Struct 1049:345
Ilic IK, Schutjajew K, Zhang W, Oschatz M (2021) J Mater Chem A 9:8711
Jaleel A, Kim S-H, Natarajan P, Gunasekar GH, Park K, Yoon S, Jung K-D (2020) J CO2 Util 35:245
Wang L, Guo J, Xiang X, Sang Y, Huang J (2020) Chem Eng J 387:124070
Antikainen R, Haapanen R, Rekolainen S (2004) J Clean Prod 12:919
Bretti C, De Stefano C, Lando G, Sammartano S (2013) Fluid Phase Equilib 355:104
Elçin S, Deligöz H (2014) Dyes Pigm 107:166
Samaddar P, Sen K (2014) J Ind Eng Chem 20:1209
Sayin S (2022) J Mol Struct 1252:132212
Elcin S, Deligöz H (2015) Sens Actuators B Chem 211:83
Pandya A, Sutariya PG, Menon SK (2013) Analyst 138:2483
Collins EM, McKervey MA, Madigan E, Moran MB, Owens M, Ferguson G, Harris SJ (1991) J Chem Soc Perkin Trans 1:3137
Gupta SC, Shivhare A, Singh D, Gupta S (2013) Def Sci J 63:442
Deligöz H, Karakuş ÖÖ (2011) Turk J Chem 35:87
Gutsche CD (1983) Acc Chem Res 16:161
Park K, Son H-J, Choe J-I (2014) J Ind Eng Chem 20:3276
Nicolescu TO (2017). In: Aliofkhazraei M (ed) Interpretation of mass spectra. Intech, London
Beklemishev M, Dmitrienko S, Isakova N (1997) Chem Anal 143:63
Boston AL, Lee EK, Surowiec K, Gega J, Bartsch RA (2012) Tetrahedron 68:8789
Puszyńska-Tuszkanow M, Daszkiewicz M, Maciejewska G, Staszak Z, Wietrzyk J, Filip B, Cieślak-Golonka M (2011) Polyhedron 30:2016
Morita Y, Agawa T, Nomura E, Taniguchi H (1992) J Org Chem 57:3658
Acknowledgements
The authors would like to thank Afyon Kocatepe University for its support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elçin, S., Gönül, R. Synthesis and characterization of a chemosensor based on trisamidetetraazocalix[4]arene linked by melamine. Monatsh Chem 154, 331–337 (2023). https://doi.org/10.1007/s00706-023-03045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03045-8