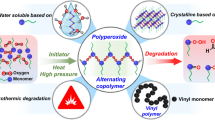
Abstract
Vinyl esters and carbonates have recently been demonstrated to have considerably lower cytotoxicity than their more commonly used (meth)acrylate counterparts, inspiring their use in the 3D printing of biomaterials. However, the degradation rates of such synthetic photopolymers are slow, especially in the mild conditions present in many biological environments. Some applications, for example, tissue regeneration scaffolds and drug release, require considerably faster biodegradation. Furthermore, it is essential to be able to easily tune the degradation rate to fit the requirements for a range of applications. Herein we present the design and synthesis of hydrolytically degradable polyphosphazenes substituted with a vinyl carbonate functionalized amino acid. Thiolene copolymerization with vinyl esters gave cured polymers which are demonstrated to considerably accelerate the degradation rates of cured vinylester/thiolene polymer scaffolds.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly(organo)phosphazenes are a family of inorganic hybrid polymers that have shown favourable biological properties in a wide range of biomedical applications [1,2,3]. Examples include drug and vaccine delivery [4, 5] and FDA-approved applications such as their use in stent linings [6, 7]. In addition, a significant property of interest for many polyphosphazenes is their (bio)degradability [2, 8, 9]. Some polyphosphazenes are susceptible to hydrolytic cleavage, the rate of which varies depending on the [5] precise nature of the substituents on the phosphorus atom in the main chain. Hence a range of degradable materials is readily accessible with degradation rates that can be tuned to suit the environment and time required of the desired application.
Amino acid ester functionalized polyphosphazenes provide an ideal basis for degradable polymer scaffolds due to the endogenous nature of the degradation products and have been widely investigated for their use in tissue regeneration applications [10,11,12,13]. Furthermore, the degradation products are reported to be pH neutral [14], a particular advantage to the more commonly polyester-based systems. Furthermore, triggered degradation pathways have also been developed, whereby the polymers were designed to be stable until exposure to specific stimuli, namely light and oxidation [15,16,17].
A field where degradability is urgently required is cured or thermoset polymers, for example, as produced in coatings or 3D printing [18] The covalent polymer networks are inherently challenging to recycle or degrade. Not only is this leading to an ever-increasing environmental burden, but for many biological applications is an outright necessity to have materials that degrade in reasonable timeframes in mild conditions. For example, established vat-photopolymerization 3D printing methods commonly exploit the fast-curing kinetics of acrylates and methacrylates, inherently producing biopersistent aliphatic carbon backbone polymers [19], even when combined with biodegradable feedstocks [20] which means that degradation of the polymer network can only proceed to a limited extent [21].
A further problem with acrylate-based chemistry is the high cytotoxicity of residual monomers due to their propensity towards Michael addition with biomolecules [22]. This problem has been resolved by the introduction of vinyl ester and viny carbonate monomers [23]. The reactivity can be improved to that similar to acrylates through the combination with thiolene addition polymerization [24,25,26]. However, these are also slowly degrading in ambient conditions with, for example, vinyl esters [23], reportedly taking many years at neutral conditions and with thiol-yne carbonates only degrading in very high or very low pH conditions [27]. Previously we have reported allyl glycine functionalized polyphosphazene [10]. These polymers were demonstrated to be outstanding scaffolds for cell growth, but the polymerization kinetics were slow. Hence in this report, we design and prepare a novel polyphosphazene with vinyl carbonate moieties and combine it with vinyl esters to give materials with degradation rates suitable for short-lifetime biomedical applications.
Results and discussion
Polydichorophosphazene [NPCl2]n (2) was first prepared from phosphine-mediated polymerization (Scheme 1) using a procedure developed in our group [28]. This reaction uses a triphenylphosphine dichloride in CH2Cl2 to initiate the living cationic polymerization of chlorophosphoranimine (1). The synthesis leads to a quantitative yield of [NPCl2]n (2), as can be readily tracked by the 31P NMR shift of the P-N backbone from about − 55 ppm to about − 18 ppm (see SI-1). The chain length of the resulting polymer is defined by the ratio of phosphine to chlorophosphoramine (1) (targeted n = 25, obtained n ≈ 23). The Mn of the polymers was estimated by 1H NMR spectroscopy of the subsequent poly(organo)phosphazene, as shown in Fig. 1.

The poly(organo)phosphazene was prepared by substituting L-serine ethyl ester onto the [NPCl2]n (2) main chain. The complete substitution was achieved at room temperature in THF, with a single peak for the poly[bis(L-serine ethyl ester)phosphazene] (SerPPz) (shift ≈ 2.5 ppm) replacing the − 18 ppm peak corresponding to [NPCl2]n (2) in the 31P NMR spectrum (Fig. 2a). A large (3 eq.) excess of the amino acid helped drive the reaction to completion. Protecting groups were not required despite the propensity of hydroxyl groups to react with [NPCl2]n (2) due to the mild conditions and higher reactivity of the amine [29]. The excess serine and triethylamine salts were removed by dialysis in anhydrous methanol, followed by reprecipitation from THF into hexanes. The SerPPz product was characterised by 1H and 31P NMR spectroscopy (see Fig. 1).
a 31P NMR spectroscopy showing the polymerization of (i) polydichorophosphazene and its complete post-polymerization functionalization with serine ethyl ester (ii) and subsequent transformation to its vinyl carbonate substituted polymer SerPPzVC (iii). b Shows the FT-IR spectra showing the final transformation of the primary alcohol on the serine moieties to the vinyl carbonates through the appearance of a band at 1650 cm−1
The free hydroxyl groups of SerPPz were then reacted with two equivalents of vinyl chloroformate to give the vinyl carbonate functionalized polyphosphazene SerPPzVC. The 1H spectrum shows the peaks of the newly introduced vinyl group in the region of 4.48–4.69 and 7.15 ppm (SI-2). Using two equivalents of vinyl chloroformates (relative to the OH groups) resulted in approximately 70% conversion of the hydroxy groups (≈1.4 of the 2 possible substituents). FT-IR spectroscopy (Fig. 2b) also demonstrated the successful functionalization with vinyl carbonates with the appearance of a band at 1650 cm−1 corresponding to the vinyl moiety.
Table 1 shows the prepared formulations. The photopolymerization of trimethylolpropane tris(3-mercaptopropionate) and divinyl adipate (DVA) (scaffold 3) have been shown to be effective in previous work and were prepared here as a reference material [1]. The radical reaction can be initiated with different initiators, such as thermal with 2,2'-azobis(2-methylpropionitrile) (AIBN) or different peroxides or with photoinitiators such as 2,2-dimethoxy-2-phenylacetophenone (DMPA). For photochemical cross-linking, ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate (TPO-L) was used as an initiator. TPO-L has been shown to have good cytocompatibility which is a significant advantage over many common initiators [30]. Figure 3b shows the FTIR confirmation of the absence of the peak of C = C stretching around 1650 ppm. The double bonds are no longer detectable for both polymerization variants chosen, again indicating a high degree of conversion. Photorheology experiments were used to characterize the photopolymerization process. A sharp increase in the storage and loss modulus is observed upon irradiation. As expected, the vinyl ester containing scaffold 3 shows faster photopolymerization kinetics than the vinyl carbonate-containing formulations (Fig. 4). The gel point was observed with the crossing of the elastic storage modulus and the viscous loss modulus and hence a high degree of conversion of the double bonds can be assumed. The same trends were observed for the thermally initiated polymerization (SI-3), where scaffold 3 revealed a vitrification point at 328 s reaction time and 180 Pa loss modulus, respectively, reflecting a higher degree of connectivity of assembled bonds as compared to scaffold 2. Scaffold 1 was not measured as it required significant amounts of solvent to form a uniform mixture with the thiol component.
Porous scaffolds were then prepared as such structures are desirable for the diffusion of cells and nutrients and thus as potential future substrates for tissue regeneration (SI-4). For this purpose, NaCl particles with an average size of 200 μm were used, and the respective mold was filled with them and then completely wetted with the polymer mixture. Afterwards, an extensive washing procedure was performed to dissolve the salt particles with water, and the scaffolds were subjected to Soxhlet extraction with ethanol overnight to eliminate residual contamination. After air drying, cylindrical pellets with approximate dimensions of 10 × 3 mm (Fig. 5) were obtained. FT-IR spectroscopy and solid-state 31P and 1H NMR spectroscopy were used to confirm the composition of the framework (SI-5).
The degradation profile at pH 7.4 and 37 °C of the prepared scaffolds are shown in Fig. 6. While the DVA-based polyester (scaffold 3) shows no sign of degradation under these conditions, scaffold 1, prepared from SerPPzVC, degraded rapidly, with 50% mass loss in only 12 days. The degradation rate of the copolymer scaffold 2, incorporating 50 mol% of DVA and 50 mol% SerPPzVC, was observed to lie between those of the pristine polymers, showing the ability of SerPPzVC to regulate the degradation process. When exposed to lower pH (pH = 2, 0.01 N HCl), a similar pattern was observed, albeit at significantly accelerated rates (SI-6), as would be expected for PPz-based polymers [31]. As the ester-based scaffold 3 didn’t degrade at all, even under these extreme conditions, it can be reasonably assumed that the P-N cleavage is responsible for the observed degradation behaviour of the SerPPzVC-based scaffolds (Fig. 6b). Upon hydrolysis of the bond between the main-chain phosphorus atom and the amino acid, the resulting hydroxyphosphazene is known to hydrolyse rapidly further, eventually to inorganic phosphates, ammonium salts, and the amino acid component as shown in Fig. 6. Non-porous scaffolds were also prepared to observe the influence of porosity on the degradation rate. As expected, the rate of mass loss is slower for the non-porous materials due to the lower surface areas, but identical trends were observed with the SerPPzVC content correlating with faster hydrolysis rates (SI-6).
Conclusion
The design and synthesis of a novel (photo)polymerisable but biodegradable polyphosphazene was described. The ethyl ester of the amino acid serine was substituted onto the phosphazene backbone to give hydrolytically cleavable polymers. By using serine as the amino acid, the free hydroxyl group could be used to decorate the PPz with curable vinyl carbonates moieties. These could be cured with multifunctional thiolene esters by thermally induced polymerization, as well as photochemically via thiolene chemistry. Porous scaffolds were prepared and the degradation profile analysed. PPz-serine polymers cured with a thiolene ester were shown to degrade at pH 7.4 and 37 °C with 50% degradation in around 12 days. The degradation at lower pH values was considerably accelerated. The DVA-thiolene polymer, used as a reference material, showed no degradation in the 60-day period or, indeed at extreme conditions of pH 2 in this timeframe. A copolymer with the DVA polymer and the PPz was observed to have degradation rates between the two, hence demonstrating that it can be used to regulate the degradation rates of such photopolymers. The chemistry developed here gives materials with potential applications as biodegradable scaffolds with fast and tunable degradation kinetics under mild conditions. The novel materials presented are interesting for further study as degradable scaffolds for tissue regeneration.
Experimental
Polymer synthesis and modification were carried out under inert conditions using a glovebox (MBRAUN) with an argon atmosphere or using standard Schlenk line techniques. For the monomer synthesis, all glassware was dried in an oven overnight at 120 °C prior to use. Celite was dried for one week also at 120 °C. The chemicals were purchased from different commercial providers. Triethylamine was purchased from Merck, distilled and stored over molecular sieves. The methanol for dialysis was also dried over a molecular sieve. Pyridine was purchased from Roth and was left to stand over KOH for several days and then distilled over CaH2. All other chemicals were purchased from commercial suppliers and used as received if not specified any differently. Photochemical reactions were carried out in a Rayonet chamber reactor with a CAMAG uv-lamp centred at 254 nm.
1H and 31P NMR spectroscopy were performed on a Brucker 300 MHz spectrometer and referenced to the signal of internal CDCl3 and MeOD. 1H NMR spectral data are given in δ/ppm relative to residual solvent peaks. 31P NMR spectral data at 121 MHz are given in δ/ppm relative to the external standard of 85% phosphoric acid. The FT-IR spectra were recorded with a Perkin Elmer Spectrum 100 FT-IR spectrometer equipped with an ATR accessory for solid-state measurements. Wavenumbers are given in \(\overline{v}/{\text{cm}}^{ - 1}\).
Scanning electron microscopy (SEM) was used to examine the morphology of the scaffolds. Samples were coated with gold for 30 s using the Sputter Coater 108. Using FEI SEM (Phenom-WorldBV) the samples were then subjected to SEM analysis in secondary electron mode using an accelerating voltage of 5 kV and a low vacuum setting.
Synthesis of trichlorophosphoranimine
The trichlorophosphoranimine monomer was synthesized using a similar approach to the literature [32]. LiN(SiMe3)2 (24.99 g, 0.15 mol) was dissolved in 500 cm3 of anhydrous diethyl ether under argon and cooled to 0 °C. Phosphorus trichloride (13.06 cm3, 0.15 mol) was added dropwise to the stirred solution within 15 min. The reaction was kept at 0 °C for 30 min and stirred for another period of 30 min at room temperature. Afterwards, thionyl chloride (12.07 cm3, 0.15 mol) was added dropwise over 15 min and the reaction mixture was again stirred as before. Then, the reaction mixture was filtered through dry Celite and the solvent was removed under reduced pressure. The final product was obtained via vacuum distillation at 4 mbar and 41 °C as a clear and colorless liquid. The monomer Cl3PNSi(CH3)3 was stored under argon at − 35 °C. Yield: 19.86 g (59%); 1H NMR (300 MHz, CDCl3): δ = 0.18 ppm (s, 9H); 31P NMR (121 MHz, CDCl3): δ = − 55.04 ppm.
Synthesis of poly(dichloro)phosphazene
The NPCl2, required for the macrosubstitution, was synthesized with modifications from literature procedures [28]. The synthesis was carried out in the glove box under an argon atmosphere. The phosphine mediator Ph3PCl2 (75 mg, 0.21 mmol) and the monomer Cl3PNSi(CH3)3 (1.2 g, 5.35 mmol) were dissolved separately in about 1 cm3 dichloromethane. Then, the monomer solution was added dropwise to the Ph3PCl2 solution and stirred for 4 h. The resulting product was used without any further purification for post polymerization. Yield: quantitative; 31P NMR (121 MHz, CDCl3): δ = − 18.20, 20.02 ppm.
Poly[bis(L-serine ethyl ester)polyphosphazene] (SerPPz)
The post-polymerization substitution was carried out according to the literature [31] with minor modifications. L-serine ethyl ester (2.72 g, 16 mmol) was suspended in 120 cm3 dry tetrahydrofuran (THF) with 15 cm3 triethylamine. The mixture was stirred at room temperature overnight and the next day refluxed for 3 h. Afterwards, the solution was filtered to remove triethylammonium chloride. The precursor (1.20 g, 5.4 mmol) was added to this mixture and stirred for 24 h. Subsequently, the polymer was filtered, concentrated, and dialyzed versus methanol for 17 h (1.0 kDa cutoff), followed by precipitation from THF into hexanes. The final product was isolated as a brittle, yellow material. Yield: 0.51 g (31%); 1H NMR (300 MHz, MeOD): δ = 1.29 (br, 3H), 3.62–3.82 (br, 2H), 4.21 (br, 3H), 7.58–7.74 (m, 15H) ppm; 31P NMR (121 MHz, MeOD): δ = 2.51 ppm; FT-IR (solid): \(\overline{v}\) = 3256 (O–H), 2980 (C–H), 1732 (C = O), 1200 (P = N) cm−1.
Vinyl carbonate functionalization of the poly[bis(L-serine ethyl ester)polyphosphazene] (SerPPzVC)
The polyphosphazene modified with the serine ethyl ester (0.45 g, 1.45 mmol) and dry pyridine (0.25 cm3, 3.06 mmol) were dissolved in 8 cm3 dichloromethane under argon atmosphere and cooled to 0 °C. Vinyl chloroformate (0.28 cm3, 3.06 mmol) was added dropwise to the stirring solution within 10 min. The reaction was kept at 0 °C for 30 min and stirred for another period for 16 h at room temperature. The reaction was hydrolyzed with 15 cm3 1 N HCl solution and the organic layer dried over MgSO4 and filtered. After evaporation of the solvent, the crude product was dissolved in methanol and purified by dialysis against methanol for 6 h (1.0 kDa cutoff). The polymer was obtained as a brown–red brittle product. Yield: 0.34 g (52%); 1H NMR (300 MHz, MeOD): δ = 1.29 (br, 3H), 3.68–3.77 (br, 2H), 4.24 (br, 3H), 4.48–4.69 (br, 2H), 7.15 (br, 1H), 7.60–7.73 (m, 15H) ppm; 31P NMR (121 MHz, MeOD): δ = 1.34 ppm. FT-IR (solid): \(\overline{v}\) = 3217 (N − H), 2926 (C − H), 1734 (C = O), 1648 (C = C), 1241 (P = N) cm−1.
Curing
Polyphosphazene (40 mg, 0.09 mmol) was dissolved in 0.1 cm3 DMF, previously degassed for 20 min with argon. Then, the initiator azobisisobutyronitrile (AIBN, 2.5 mg, 4 wt%), the crosslinker DVA (17 mm3, 0.09 mmol) and trithiol (40 mm3, 0.12 mmol) were added and the mixture was shaken until a homogenous mixture was obtained. Finally, the solution was filled into a 4 cm3 glass vial and put into the oven at 65 °C for about 3 h. For the pore generation, NaCl was put into the vial equally distributed across the well to wet each part. For the photochemical pathway instead AIBN, the photoinitiator ethyl (2,4,6-trimethylbenzoyl) phenyl phosphinate (TPO-L, 1 wt%) was added under exclusion of light to the polymer solution and homogeneously mixed. The sample was placed for 1 h in the UV chamber at 254 nm and at a temperature of 4 °C.
Afterward, the vials were placed upside down on crystallizing dishes filled with distilled water for 45 min to enable the heavier salt to sink to the bottom. Subsequently, the pellets were carefully removed from the vials with a fine spatula. Then, they were cleaned with a Soxhlet extraction with ethanol for 16 h and afterwards were dried on a filter paper leading a porous scaffold. Scaffold 2: 31P NMR (solid): δ = − 8.95, 0.21 ppm; 13C NMR (solid): δ = 7.98 (CH3), 14.77 (CH-CH3), 24.87–35.35 (CH2), 41.57 (C-S), 54.18 (NH-CH), 64.08 (OCH2), 171.83–173.38 (C = O) ppm; FT-IR (solid): \(\overline{v}\) = 3181 (O–H), 2924 (C-H), 1731 (C = O), 1223 (P = N) cm−1.
Rheology experiments
The formulations for rheology were prepared by dissolving SerPPzVC, DVA, and trithiol (approximate total amount of 120 mg) and TPO-L (1 wt%) in 0.1 cm3 DMF. Photoreactivity was performed using an Anton Paar MCR 502 rheometer equipped with a thermostatized optical detection plate and by adding a self-developed adapter and a UV source (High-power UV-led solo P). Storage and loss modulus were measured by using UV light with a wavelength of 254 nm. The sample was irradiated with a light intensity of 0.25 W cm−2 in a plate-plate geometry (d = 10 mm) at room temperature. Thermal polymerization was carried out with AIBN instead of TPO-L at a temperature of 85 °C. The measurement was made in oscillation mode using two steps, where the frequency changed from 15 to 10 Hz (and γ from 10 to 1%) at 100 Pa (G´) to stay within the linear viscoelastic region.
Degradation studies
To determine the degradation of the polymers, the loss of mass was controlled. For this purpose, an average of 50 mg of the scaffold was weighed in a sealed vial and placed in 2 cm3 pure MilliQ water (pH 7.4) or in 0.01 N HCl (pH 2) at 37 °C. The observation period is two months for samples at pH 7.4 and just under one month for samples at pH 2 carried out in triplicate at a regular time. The scaffolds were filtered through dried to mass constancy glass sintered crucibles and dried in a vacuum oven at 50 °C. The mass loss was determined gravimetrically.
Data availability
Authors can confirm that all relevant data are included in the article and/or its supplementary information files.
References
Rothemund S, Teasdale I (2016) Chem Soc Rev 45:5200
Allcock HR, Morozowich NL (2012) Polym Chem 3:578
Teasdale I, Brüggemann O, Henke H (2020) Polyphosphazenes for medical applications. De Gruyter
Eng NF, Garlapati S, Gerdts V, Potter A, Babiuk LA, Mutwiri GK (2010) Curr Drug Delivery 7:13
Andrianov AK, Langer R (2021) J Controlled Release 329:299
Hiroyuki J, Hiroyoshi M, Qi C, Matthew K, Sho T, Atsushi S, Liang G, Eduardo A, Anuj G, Frank DK, Renu V, Aloke VF (2019) EuroIntervention 15:e342
Bates MC, Yousaf A, Sun L, Barakat M, Kueller A (2019) Regener Eng Transl Med 5:341
Brito J, Andrianov AK, Sukhishvili SA (2022) ACS Appl Bio Mater 5:5057
Henke H, Wilfert S, Iturmendi A, Brüggemann O, Teasdale I (2013) J Polym Sci A: Polym Chem 51:4467
Rothemund S, Aigner TB, Iturmendi A, Rigau M, Husár B, Hildner F, Oberbauer E, Prambauer M, Olawale G, Forstner R, Liska R, Schröder KR, Brüggemann O, Teasdale I (2015) Macromol Biosci 15:351
El-Amin SF, Kwon MS, Starnes T, Allcock HR, Laurencin CT (2006) J Inorg Organomet Polym 16:387
Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT (2011) Adv Funct Mater 21:2641
Peach MS, Kumbar SG, James R, Toti US, Balasubramaniam D, Deng M, Ulery B, Mazzocca AD, McCarthy MB, Morozowich NL, Allcock HR, Laurencin CT (2012) J Biomed Nanotechnol 8:107
Deng M, Kumbar SG, Wan Y, Toti US, Allcock HR, Laurencin CT (2010) Soft Matter 6:3119
Teasdale I (2019) Eur J Inorg Chem 2019:1445
Bouché M, Pühringer M, Iturmendi A, Amirshaghaghi A, Tsourkas A, Teasdale I, Cormode DP (2019) ACS Appl Mater Interfaces 11:28648
Iturmendi A, Theis S, Maderegger D, Monkowius U, Teasdale I (2018) Macromol Rapid Commun 39:1800377
Voet VSD, Guit J, Loos K (2021) Macromol Rapid Commun 42:e2000475
Muralidharan A, McLeod RR, Bryant SJ (2022) Adv Funct Mater 32:2106509
van Hoorick J, Tytgat L, Dobos A, Ottevaere H, van Erps J, Thienpont H, Ovsianikov A, Dubruel P, van Vlierberghe S (2019) Acta Biomater 97:46
Voet VSD, Guit J, Loos K (2021) Macromol Rapid Commun 42:2000475
Heller C, Schwentenwein M, Russmueller G, Varga F, Stampfl J, Liska R (2009) J Polym Sci A: Polym Chem 47:6941
Husár B, Heller C, Schwentenwein M, Mautner A, Varga F, Koch T, Stampfl J, Liska R (2011) J Polym Sci A: Polym Chem 49:4927
Mostegel FH, Roth M, Gassner M, Oesterreicher A, Piock R, Edler M, Griesser T (2016) Prog Org Coat 94:116
Mautner A, Qin X, Kapeller B, Russmueller G, Koch T, Stampfl J, Liska R (2012) Macromol Rapid Commun 33:2046
Mautner A, Qin X, Wutzel H, Ligon SC, Kapeller B, Moser D, Russmueller G, Stampfl J, Liska R (2013) J Polym Sci A: Polym Chem 51:203
Wu Y, Simpson MC, Jin J (2021) Macromol Chem Phys 222:2000435
Wilfert S, Henke H, Schoefberger W, Brüggemann O, Teasdale I (2014) Macromol Rapid Commun 35:1135
Krogman NR, Weikel AL, Nguyen NQ, Nair LS, Laurencin CT, Allcock HR (2008) Macromolecules 41:7824
Kim G-T, Go H-B, Yu J-H, Yang S-Y, Kim K-M, Choi S-H, Kwon J-S (2022) Polymers 14:979
Linhardt A, König M, Iturmendi A, Henke H, Brüggemann O, Teasdale I (2018) Ind Eng Chem Res 57:3602
Wang B, Rivard E, Manners I (2002) Inorg Chem 41:1690
Acknowledgements
We are grateful to Dr Claudia Schröder for her support with this work.
Funding
Open access funding provided by Johannes Kepler University Linz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ajvazi, E., Bauer, F., Kracalik, M. et al. Poly[bis(serine ethyl ester)phosphazene] regulates the degradation rates of vinyl ester photopolymers. Monatsh Chem 154, 489–496 (2023). https://doi.org/10.1007/s00706-023-03042-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03042-x