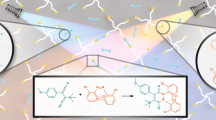
Abstract
The synthesis of two dye-labeled azides via de-symmetrization of 2,6-bis(4-azidobenzylidene)-4-methylcyclohexanone (BAC-M) with a copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) using fluorescent dyes is reported. An alkyne functionalized dansyl derivative and an alkyne functionalized perylene diimide derivative were used as the dyes. The photo-physical properties of these dye dyads are described, and their performance in multi-photon grafting onto polyethylene glycol-based hydrogels is investigated. While the dansyl-conjugated BAC derivate is well suited for multi-photon grafting with lasers operating at 800 nm, the perylene diimide-bearing dye does not give the desired result.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic azides are versatile compounds with widespread use in organic synthetic chemistry [1] as well as in material science [2]. In particular, organic azides are used in 1,3-dipolar cycloaddition reactions as dipoles, e.g., with alkynes yielding 1,2,3-triazoles, the so-called Huisgen reaction. This reaction gained a lot of interest in the field of bio-conjugation, particularly in the form of their copper-catalyzed variant (CuAAC) [3] and their copper-free variant using strained alkynes (SPAAC) [4]. Another reactivity of organic azides is their propensity to form nitrenes and nitrogen upon thermal or photochemical activation. The nitrenes are highly reactive and show manifold reaction pathways [5]. Most importantly, nitrenes can easily insert into C–H bonds yielding amines, a reactivity exploited for the post-polymerization functionalization and crosslinking of polymers [2]. Historically, resin formulations based on organic bis-azides dispersed in rubbers were first disclosed by Eastman Kodak [6] and were used as photoresists operating with deep UV light (λEx < 300 nm) in semiconductor chemistry in the early days. Nowadays, negative resists operating at a wavelength of 365 nm are available [2]. About 10 years ago, the feasibility of using multi-photon excitation for performing grafting with bis-azides was demonstrated [7]. Due to the confinement of the photoreaction within the focal volume of the laser beam, multi-photon grafting (MPG) provides excellent spatial control over traditional photo-grafting. Moreover, the use of NIR pulsed laser (∼800 nm) as excitation source, which causes less photo-damage and unwanted side reactions, allows for direct 3D photo-grafting within transparent materials. The first example of MPG was reported by Liska et al. using the commercial aromatic azide 2,6-bis(4-azidobenzylidene)-4-methylcyclohexanone (BAC-M) as the grafting agent. BAC-M is characterized by a high three-photon absorption cross section of 1.19 × 10−78 cm6 s2 at 800 nm. Under NIR pulsed laser excitation, BAC-M decomposed and the formed active nitrene species reacted at the defined locations within the 3D matrix with a high resolution of about 4 μm [7]. Considering the lower probability of three-photon absorption usually requiring higher laser intensity, the two-photon grafting agent 4-[(4-azido-2,3,5,6-tetrafluorobenzylidene)amino]-N,N-dimethylaniline (AFA) containing a C = N bond as a π-bridge, a N-methylaniline moiety as an electron donor, and a fluorinated aromatic azide as the electron acceptor was constructed. Fluorination drastically suppressed the ring expansion deactivation of the singlet nitrene [2], resulting in high photo-grafting efficiency. AFA exhibits a two-photon absorption cross section of 178 GM at 800 nm in DMF solution, which is sufficient for MPG. As compared to BAC-M, AFA requires a lower laser intensity to induce MPG, therefore enabling a larger processing window and a higher writing speed in photo-grafting process [8]. Based on AFA, a fluoroaryl azide with terminal alkyne groups, namely 4-[(4-azido-2,3,5,6-tetrafluorobenzylidene)-amino]-N-methyl-N-(prop-2-yn-1-yl)aniline (AFA-3), was developed. AFA-3 allows for MPA grafting of an alkyne functionality, which subsequently serves as the reaction partner in the CuAAC “click” reaction [9]. Thus, generated 3D micro-patterns can be further functionalized, e.g., with dye molecules (Fig. 1).
Despite of the promising potential of aromatic azides, their use for MPG in biological applications that require aqueous conditions is limited by their hydrophobic nature. Very recently, a commercially available hydrophilic azide, namely 4,4′-diazido-2,2′-stilbenedisulfonic acid (DSSA) sodium salt, was successfully applied to MPG to alter the chemical and physical properties of cell-laden hydrogel matrix, leading to the alignment and directional migration of cells. Considering the relatively short conjugated length of DSSA, an uncommonly excitation wavelengths of 700 and 725 nm were required to ensure efficient MPG in this case [10]. In summary, the vast chemical and functional space of aromatic azides for MPG remains to be explored. However, to synthesize azides possessing large two-photon absorption at desired wavelength is still a great challenge due to the high sensitivity and thermal instability of azides.
Herein we present the synthesis of two aromatic azides based on coupling a fluorescent dye with one azide group of a photoactive aromatic bis-azide (Fig. 1). Furthermore, we disclose their photo-physical properties and evaluate their performance in two-photon grafting onto polyethylene glycol-based hydrogels. Although previously used BAC-M yields upon grafting luminescent groups [11], the deliberate installment of chromophores is desirable. Two dyes were used for demonstrating this concept. Both exhibit much higher quantum yields than the chromophore resulting from BAC-M grafting, and the dansyl dye is, in terms of its solvatochromic properties, also functional [12].
Results and discussion
The perylene-based dye bearing an alkyne group was prepared by a one-pot reaction [13] of 1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic bis-anhydride (TCP) with 2-ethylhexylamine and prop-2-yn-1-amine and separation of the desired asymmetrically substituted perylene diimide derivative 1 by column chromatography in 31% yield (Scheme 1). Attempts to use more elaborated synthetic methods toward asymmetrically substituted perylene diimides [14, 15] with TCP as the precursor failed in terms that low yields of 1 were obtained. TCP was selected as the starting material because it shows better solubility than the parent perylene derivative and offers the possibility for further functionalization through the chloro-atoms in the so-called bay region [16]. The swallow-tailed 2-ethylhexylamine was used to further improve the solubility of the perylene dye [14]. Compound 1 shows characteristic signals for the methylene groups next to the imide nitrogen in the 1H NMR spectrum. The prop-2-yn-1-amine-derived methylene group gives a singlet at 4.99 ppm, and the 2-ethylhexylamine substituent is characterized by a multiplet at 4.15 ppm. Furthermore, the alkyne proton gives rise to a singlet at 2.23 ppm.

In the next step, 1 was reacted with a fourfold excess of 2,6-bis(4-azidobenzylidene)-4-methylcyclohexanone (BAC-M) in the presence of catalytic amount of copper(I) iodide using dichloromethane as the solvent. The desired compound 2 was finally obtained upon column chromatographic purification in 52% yield and is characterized by a singlet at 8.16 ppm in the 1H NMR spectrum assigned to the newly formed tri-azole proton. The intensity of this signal is one-third when compared to the signal stemming from the methyl group of the BAC-M part of the molecule giving a doublet at 1.10 ppm. Furthermore, infrared spectroscopy revealed the presence of the azide group by showing an absorption peaking at 2117 cm−1.
The dansyl-based dye 4 was prepared in similar way as 2. Upon reacting alkyne 3 with a tenfold excess of BAC-M as the starting materials, 4 was obtained in 76% yield (Scheme 2). Characteristically, the newly formed triazole proton gives rise to a singlet at 7.75 ppm in the 1H NMR spectrum, which exhibits, compared to the methyl group of BAC-M, a relative intensity of 1. Again, the presence of the azide was confirmed by infrared spectroscopy.

The optical properties of 2 and 4 were assessed by absorption and emission measurements in dichloromethane. UV–VIS spectra of 2 showed an absorption maximum at 520 nm with a molar attenuation coefficient (ε) of 34000 dm3 mol−1 cm−1 and the for perylenes typical vibronic progression [17]. Furthermore, the absorption of the BAC-M part is peaking at 343 nm (ε = 21000 dm3 mol−1 cm−1). Compared to parent BAC-M, this absorption is hypsochromically shifted by 14 nm, while the perylene-related spectral features in 1 and 2 are the same (Fig. 2).
The emission of 2 (as well as of 1) is peaking at 550 nm (Fig. 3). For excitation, light with a wavelength of 519 nm was used (excitation spectra of 1 and 2 are very similar). The UV–VIS spectrum of 4 is dominated by two features, one peaking at 255 nm the other at 347 nm. The latter absorption is a sum of the absorption of the dansyl dye and the BAC-M and exhibits a ε of 20000 dm3 mol−1 cm−1 (Fig. 2). Fluorescence of 4 and its precursor 3 is peaking at 507 nm (λex = 345 nm) and the excitation spectra of 3 and 4 are very similar (Fig. 3).
Two-photon cross sections (σTPA800) at 800 nm of 1, 2, and 4 were measured using the Z-scan technique in chloroform solution (c = 1.0 × 10–3 mol dm−3) at different laser powers (Fig. 4a) and calculated according to Sheik–Bahae [18]. The linear scaling of the relative absorption (expressed as q0) with pulse energy suggests that no exited-state absorbance occurs in these cases (Fig. 4b). For the alkyne derivative 1, a σTPA800 of 55 GM (1 GM = 10−50 cm4 s photon−1 molecule−1) was obtained, which is 2.5-fold higher than it was found for a related symmetric 1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic diimide [19]. Azide 2 exhibited a somewhat higher σTPA800 of 72 GM and azide 4 is characterized by the highest σTPA800 of 100 GM obtained in this study. In contrast, the symmetric bis-azide BAC-M shows multi-photon absorption at 800 nm characterized by a three-photon absorption cross section of 1.19 × 10−78 cm6 s2 [8, 20]. Accordingly, de-symmetrization of the BAC-M core by transforming one azide group into a tri-azole group is held responsible for obtaining two-photon absorption in the molecules introduced here. This is particularly illustrative in the case of mono-azide 4. Its precursor 3 was found to exhibit a small two-photon cross section of below 10 GM as estimated from two-photon-induced emission measurements against rhodamine [21, 22]. Because the dansyl-chromophore 3 is regarded to be electronically decoupled from the BAC-M chromophore, the main contribution of the σTPA800 of 4 is likely stemming from the de-symmetrized BAC-M chromophore.
a experimental Z-scan data (dots) and fitting curves (full lines) for 2 using pulse energies from 75 to 135 nJ; b linear correlation of the rel. absorption q0 and the laser power as obtained for 2; c Laser scanning micrograph of patterns obtained by two-photon-induced photo-grafting of 4 (structure above) showing an array of squares produced at different laser power (290-40 mW; step = − 50 mW) and different scanning velocities (1–5 mm s−1)
Compounds 2 and 4 were then used for photo-grafting of a poly(ethylene glycol)diacrylate-based hydrogel swollen in DMF solutions of the dyes (2 wt%). Upon laser writing, a series of woodpile patterns were produced at a scanning velocity of 1000–5000 μm s−1 with an average laser power values in the range of 40–290 mW. The patterns are obtained by moving the sample position relatively to the laser focus in 3D using three linear translation stages in a layer-by-layer fashion. After grafting, not grafted components were removed by washing with DMF and the outcome of the process was then visualized with the aid of laser scanning microscopy. The corresponding micrograph of the array of woodpile patterns (in the size of 50 × 50 × 40 µm) obtained with compound 4 is shown in Fig. 4c. Fluorescence signal for the structures produced at a lower end of the average laser power is weaker, indicating that the efficiency of the grafting process, i.e., the concentration of immobilized molecules, depends on the irradiation dose. Please note that the pattern obtained with the highest laser power and the lowest speed is not accurately formed because the laser was not operating properly during fabrication of the corresponding pattern. In contrast, with the other compound 2, no such pattern could be obtained. Doubling the concentration of the dye in DMF did not improve the outcome, instead also some decomposition of the matrix was observed visually but was not further investigated. Essentially, 2 is not suited for two-photon grafting under the selected conditions. A possible explanation for the failure of 2 might be found in the photo-physical properties of this molecule. It is conceivable that efficient energy transfer from the excited BAC-M part to the perylene diimide part occurs, impeding the desired photochemical nitrene formation.
Conclusion
We herein described the access to two fluorescent dyes bearing an aromatic azide group. The syntheses rely on the de-symmetrization of a commercially available aromatic bis-azide derivative by performing copper(I)-catalyzed alkyne-azide cycloaddition with corresponding alkyne functionalized dyes. A dansyl and a perylene diimide-based dye derivate was used for the conjugation, and two-photon cross sections at 800 nm obtained from Z-scan measurements in the range of 70–100 GM were obtained for the two dye-dyads under investigation. Thus, de-symmetrization of the tri-photon absorbing symmetric bis-azide led to two-photon absorbing materials with respectable cross sections. The newly synthesized dyes were then tested in photo-grafting of a poly-ethylene-glycol-based hydrogel, and in case of dansyl-based dye dyad, photo-grafting was feasible. In contrast, the perylene diimide-based photo-grafting agent failed in this application.
Experimental
All chemicals used for this work were purchased from commercial sources (Fluka, Sigma Aldrich, Lancaster, Merck or ABCR) and unless mentioned otherwise, applied without further purification. 2,6-Bis(4-azidobenzylidene)-4-methylcyclohexanone (BAC-M) was obtained from Sigma-Aldrich and 1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic bis-anhydride (TCP, technical grade) was purchased from Beijing Wenhaiyang Industry and Trading Co. Ltd. 5-(Dimethylamino)-N-(prop-2-yn-1-yl)naphthalene-1-sulfonamide (3) was prepared according to the literature [23]. Analytical thin-layer chromatography (TLC) was performed on Merck silica gel 60-F254, and spots were visualized by UV light or by treatment with aqueous potassium permanganate (0.5 w%). Column chromatography was performed using silica gel 60 Å from Acros Organics. NMR spectra were recorded on a Bruker Avance III 300 MHz FT NMR spectrometer (300.36 MHz (1H), 75.53 MHz (13C)). Deuterated solvents were purchased from Cambridge Isotope Laboratories, Inc., Chemical shifts δ [ppm] are referenced to residual protonated solvent signals as internal standard CDCl3: δ = 7.26 ppm (1H), 77.16 ppm (13C). The following abbreviations were taken to indicate different peak shapes: s (singlet), d (doublet), t (triplet), q (quadruplet), m (multiplet), b (broad), bs (broad singlet), dd (doublet–doublet). Elemental analyses were conducted on an Elementar Vario Micro Cube with CHN detector. Results were found to be in good agreement (± 0.3%) with calculated values. For IR spectroscopy, a Bruker ALPHA FT-IR Spectrometer was used. Measurements were performed in ATR mode. UV–VIS absorption spectra were taken with a Cary 50 UV–VIS Spectrophotometer from Varian and fluorescence spectra on a Perkin Elmer Luminescence Spectrometer LS50B. The measurements were done with a silica glass bulb applicative for a spectral range from 300 to 800 nm from Hellma. Open aperture Z-scan analyses were made with a Ti:sapphire laser system (25 fs pulse duration, 1 kHz repetition rate at 798 nm). A detailed description of the set characterization can be found elsewhere [24]. Sample solutions (concentration of 1.0 × 10–3 mol dm−3) in spectroscopic grade tri-chloromethane (CHCl3) were measured in a 0.2 mm thick flow cell in a non-recycling volumetric flow of 4.0 cm3 h−1. The measurements were carried out at different pulse energies ranging from 50 to 135 nJ. The experimental data were fitted using the adopted equations of Sheik-Bahae et al. to obtain the two-photon cross section (σTPA) [18].
Two-photon grafting was demonstrated on samples prepared from poly(ethylene glycol) diacrylate (average molecular weight 700 g mol−1, Sigma Aldrich) dissolved in dimethyl-formamide (DMF, ratio 50:50) upon photo-polymerization. As the photo-initiator Irgacure 819 (1 wt%, Ciba Specialty Chemicals Inc.) was used and upon irradiation with UV light for 5 min (Intelliray 600), the formulation was cured in silicone molds (diameter 6 mm; thickness 1 mm). For laser photo-grafting, the samples were immersed into the solution of 2 or 4 in DMF (2–4 w% of the dye). A Ti:sapphire femtosecond laser (Femtotrain, HighQ), emitting pulses with duration of 80 fs at a 73 MHz repetition rate around 793 nm was used for laser grafting. The laser beam was focused with a 20 × microscope objective (Zeiss, NA = 0.8) into the sample. An acousto-optical modulator was used for fast switching of the laser beam and for adjusting its intensity. The laser power was measured before the objective. The patterns were obtained by scanning the focused laser beam within the sample by the galvo-scanner (HurryScan, ScanLab). In processing window tests, the grafted square patterns were produced by a set of adjacent line scans. The inscribed structures had a size of 50 × 50 × 40 µm with a coating pitch of 0.5 µm. The line arrays were grafted with different laser power (40–290 mW) and different scanning speed (1–5 mm s−1). After the grafting procedure, the samples were placed in DMF to remove the residual dyes. The fluorescence of the patterned samples was analyzed by laser scanning microscopy (LSM700 ZEN software, Carl-Zeiss) at the excitation wavelength of 555 nm.
1,6,7,12-Tetrachloroperylene-3,4,9,10-tetracarboxylic-2-(2-ethylhexyl)-8-(prop-2-yn-1-yl)diamine (1, C35H24Cl4N2O4)
To a dispersion of 430 mg 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic bis-anhydride (0.811 mmol) in 5 cm3 toluene 0.146 cm3 2-ethylhexan-1-amine (0.892 mmol) and 0.057 cm3 prop-2-yn-1-amine (0.892 mmol) were added. The reaction mixture was stirred for 24 h at 110 °C. Volatiles were removed in vacuum and the residue was purified by column chromatography (CH2Cl2/pentane = 1/3) giving upon drying 170 mg (31%) of the desired product. Rf (CH2Cl2) = 0.43; 1H NMR (300 MHz, CDCl3, 25 °C): δ = 8.71, 8.69 (s, 4H, per2,5,8,11), 4.99 (s, 2H, N-CH2-C≡CH), 4.15 (m, 2H, N-CH2-CH), 2.23 (s, 1H, N-CH2-C≡CH), 1.92 (m, 1H, N-CH2-CH), 1.50–1.25 (m, 8H), 0.98–0.75 (m, 6H) ppm; 13C{1H} NMR (75 MHz, CDCl3, 25 °C): δ = 162.6, 162.5 (4C, C = O), 135.6, 135.4, 133.3, 133.1, 131.6, 131.4, 129.1, 128.4, 123.4, 122.8 (20C, per), 77.8 (1C, CH2-C≡CH), 71.3 (1C, CH2-C≡CH), 44.6 (1C, N-CH2-CH), 38.0 (1C, N-CH2-CH), 30.7 (1C, CH2-C≡CH), 29.9, 28.7, 24.0, 23.1, 14.1, 10.6 (6C, alkyl) ppm; UV–Vis (CH2Cl2, c = 2.6∙10–3 mol dm−3): λmax (ε) = 520 (27000), 485 (18500), 426 (6000) nm (mol−1 dm3 cm−1); fluorescence (CH2Cl2, c = 2.3∙10–4 mol dm−3, λex = 519 nm): λem = 550 nm.
2-[[1-[4-[(E)-[3-((E)-4-Azidobenzylidene)-5-methyl-2-oxocyclohexylidene]methyl]phenyl]-1H-1,2,3-triazol-4-yl]methyl]- 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic-2-(2-ethylhexyl)-8-(prop-2-yn-1-yl)diamine (2, C56H42Cl4N8O5)
To a dispersion of 100 mg 1 (0.148 mmol) and 200 mg BAC-M (0.592 mmol) in 7 cm3 CH2Cl2 1.5 mg copper iodide (0.0074 mmol) was added and the reaction mixture was stirred for 72 h at 35 °C in the dark. Afterward, the reaction mixture was diluted with 30 cm3 CH2Cl2 extracted with water (3 × 50 cm3). The organic phase was dried over Na2SO4 and the volatiles were removed in vacuum. The obtained residue was purified by column chromatography (CH2Cl2/pentane = 1/3 changing to CH2Cl2 changing to CH2Cl2/Methanol = 20/1) giving upon drying 80 mg (52%) of 2. Rf (CH2Cl2/methanol = 20/1) = 0.41; 1H NMR (300 MHz, CDCl3, 25 °C): δ = 8.71, 8.69 (s, 4H, per2,5,8,11), 8.16 (s, 1H, tri-azole), 7.77, (m, 4H, Ph, C = CH-), 7.57, 7.47, 7.06 (d, 6H, Ph), 5.63 (s, 2H, N-CH2-tri-azole), 4.12 (m, 2H, N-CH2-CH), 3.03 (m, 2H, BAC-M), 2,52 (m, 2H, BAC-M), 1.94 (m, 2H, BAC-M, N-CH2-CH), 1.50–1.27 (m, 8H), 1.10 (d, 3H, BAC-M), 0.98–0.78 (m, 6H) ppm; 13C{1H} NMR (75 MHz, CDCl3, 25 °C): δ = 188.5 (1C, C = OBAC-M), 161.5, 161.4 (4C, C = Oper), 142.7, 139.5, 134.5, 134.3, 134.2, 133.7, 131.1, 130.4, 129.9, 127.9, 127.4, 122.32, 122.26, 121.9, 120.5, 119.3, 118.1 (38 C, per, tri-azole, BAC-M), 44.6 (1C, N-CH2-CH), 43.6 (1C, N-CH2-tri-azole), 37,7 (1C, N-CH2-CH), 37.0, 35.5, 35.4, 29.7, 29.3, 28.2, 27.6, 20.6, 13.1, 9.9 (10C, BAC-M, alkyl) ppm; IR (Nujol): \(\overline{V}\) = 2117 (azide) cm−1; UV–Vis (CH2Cl2, c = 2.9∙10–3 mol dm−3): λmax (ε) = 520 (34000), 485 (23500), 426 (10000), 343 (21000) nm (mol−1 dm3 cm−1); fluorescence (CH2Cl2, c = 2.0∙10–4 mol dm−3, λex = 519 nm): λem = 550 nm.
N-[[1-[4-[(E)-[3-((E)-4-azidobenzylidene)-5-methyl-2-oxocyclohexylidene]methyl]phenyl]-1H-1,2,3-triazol-4-yl]methyl]-5-(dimethylamino)naphthalene-1-sulfonamide (4, C36H34N8O3S)
A solution of 1.00 g BAC-M (2.70 mmol) and 78 mg 3 (0.270 mmol) in 20 cm3 CH2Cl2 (abs.) was stirred for 10 min at room temperature. Subsequently, 38 mg copper(I) iodide (0.135 mmol) was added to the yellow solution and the reaction mixture was heated to 30 °C for 120 h. Afterward, the reaction mixture was poured into 50 cm3 water, and the organic phase was extracted with CH2Cl2 (3 × 20 cm3). The combined organic phases were dried over Na2SO4 and the solvent evaporated under reduced pressure. The residue was purified by column chromatography (cyclohexane/ethyl acetate = 5/1) giving 135.2 mg (76%) of 4. Rf (cyclohexane/ethyl acetate = 5/1) = 0.49; 1H NMR (300 MHz, CDCl3, 25 °C): δ = 8.49 (d, 1H, nap4), 8.31, 8.30 (d, 2H, nap2,8), 7.75 (s, 1H, tri-azole), 7.60–7.42 (m, 10H, ph2,2′,3,5,6,6′, nap2,7, C = CH-), 7.15 (d, 1H, naph6), 7.08 (d, 2H, ph3′,5′), 5.86 (t, 1H, NH), 4.33 (d, 2H, NHCH2-tri-azole), 3.02 (d, 2H, BAC-M), 2.82 (s, 6H, NCH3), 2.52 (m, 2H, BAC-M), 2.04 (bm, 1H, BAC-M), 1.11 (d, 3H, BAC-M) ppm; 13C{1H} NMR (75 MHz, CDCl3, 25 °C): δ = 189.7 (1C, C = O), 144.8, 140.7, 136.9, 136.6, 136.3, 135.4, 134.9, 134.8, 132.7, 132.3, 131.7, 130.8, 130.0, 128.8, 123.5, 120.2, 119.2, 118.4, 115.6, 114.4 (24 C, nap, tri-azole, BAC-M), 45.6 (2C, NCH3), 38.9 (1C, NH-CH2-tri-azole), 36.7 (2C, BAC-M), 29.8 (1C, BAC-M), 21.8 (1C, BAC-M) ppm; IR (Nujol): \(\overline{V}\) = 2117 (azide) cm−1; UV–Vis (CH2Cl2, c = 4.1∙10–3 mol dm−3): λmax (ε) = 347 (20000), 255 (23000) nm (mol−1 dm3 cm−1); fluorescence (CH2Cl2, c = 4.0∙10–4 mol dm−3, λex = 345 nm): λem = 507 nm.
Data availability
The data that support the findings of this study are available from the corresponding author, CS, upon reasonable request.
References
Bräse S, Gil C, Knepper K, Zimmermann V (2005) Angew Chem Int Ed 44:5188
Schock M, Bräse S (2020) Molecules 25:1009
Heina JE, Fokin VV (2010) Chem Soc Rev 39:1302
Kim E, Koo H (2019) Chem Sci 10:7835
Wang Y-C, Lai X-J, Huang K, Yadav S, Qiu G, Zhang L, Zhou H (2021) Org Chem Front 8:1677
Hepher M, Wagner HM (1958) Azide resin photolithographic compositions. US Patent 2852379 A, Sept 16, 1958; (1959). Chem Abstr 53:5482
Ovsianikov A, Li Z, Torgersen J, Stampfl J, Liska R (2012) Adv Funct Mater 22:3429
Li Z, Ajami A, Stankevicius E, Husinsky W, Raciukaitis G, Stampfl J, Liska R, Ovsianikov A (2013) Opt Mater 35:1846
Li Z, Stankevicius E, Ajami A, Raciukaitis G, Husinsky W, Ovsianikov A, Stampfl J, Liska R (2013) Chem Commun 49:7635
Sayer S, Zandrini T, Markovic M, Van Hoorick J, Van Vlierberghe S, Baudis S, Holnthoner A, Ovsianikov A (2022) Sci Rep 12:8626
Eisenhart JM, Ellis AB (1985) J Org Chem 50:4108
Lum K, Zielinski SM, Abelt CJ (2021) J Phys Chem A 125:1229
Gallas K, Knall A-C, Scheicher SR, Fast DE, Saf R, Slugovc C (2014) Macromol Chem Phys 215:76
Wicklein A, Lang A, Muth M, Thelakkat M (2009) J Am Chem Soc 131:14442
Huang C, Potscavage WJ, Tiwari SP, Sutcu S, Barlow S, Kippelen B, Marder SR (2012) Polym Chem 3:2996
Aigner D, Borisov SM, Petritsch P, Klimant I (2013) Chem Commun 49:2139
Leroy-Lhez S, Baffreau J, Perrin L, Levillain E, Allain M, Blesa M-J, Hudhomme P (2005) J Org Chem 70:6313
Sheik-Bahae M, Said AA, Wei T-H, Hagan DJ, Van Stryland EW (1990) IEEE J Quantum Electron 26:760
Mariz IFA, Raja S, Suzete TS, Torres AE, Baleizão C, Maçôas E (2021) Dyes Pigm 193:109470
Ovsianikov A, Li Z, Ajami A, Torgersen J, Husinsky W, Stampfl J, Liska R (2012) Appl Phys A 108:29
Chui C-H, Wang Q, Chow W-C, Yuen MC-W, Wong KL, Kwok M-W, Cheng GY-M, Wong RS-M, Tong SW, Chan K-W, Lau F-Y, Lai PB-S, Lam K-H, Fabbri E, Tao X-M, Gambari R, Wong W-Y (2010) Chem Commun 46:3538
Xu C, Zipfel W, Shear JB, Williams RM, Webb WW (1996) Proc Natl Acad Sci USA 93:10763
Bolletta F, Fabbri D, Lombardo M, Prodi L, Trombini C, Zaccheroni N (1996) Organometallics 15:2415
Ajami A, Husinsky W, Liska R, Pucher N (2010) J Opt Soc Am B 27:2290
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Open access funding provided by Graz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallas, K., Wohlmuth, D., Li, Z. et al. Dye-labeled aromatic azides for multi-photon grafting. Monatsh Chem 154, 481–488 (2023). https://doi.org/10.1007/s00706-022-03022-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-03022-7