Abstract
A series of substituted imidazoquinolines, a structurally related chemotype to pyrazoloquinolinones, a well-known class of GABAA ligands, was prepared via two synthetic procedures and the efficiency of these procedures were compared. One method relies on classical heterocyclic synthesis, the other one aims at late-stage decoration of a truncated scaffold via direct C–H functionalization. A pharmacological evaluation disclosed that one of the synthesized derivatives showed interesting activity on a α1β3 containing receptor subtype.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The endogenous neurotransmitter, γ-aminobutyric acid (GABA), plays a major role in neurotransmission in the mammalian brain, where it binds to ligand-gated ion channel type A receptors (GABAA) [1, 2]. These pentameric receptors are composed of different subunits (α1-6, β1-3, γ1-3, δ, ε, π, θ, ρ1–3) and the specific assemblies thereof (receptor subtype) determine their pharmacological effects [3]. Upon GABA binding, an anion flux is induced, which can be affected by a variety of allosteric modulators. This is of clinical importance in various ways. For example, it has been reported that sedative and anxiolytic effects are elicited by allosteric modulators (such as benzodiazepines), depending on the particular receptor subtype [4].
Allosteric modulators which use a large number of allosteric sites [5] are in widespread use as anaesthetics, anticonvulsants, sedatives, and tranquilizers [6, 7]. Moreover, they are known to bind to the α + /γ- interfaces of the pentameric receptors [8]. Nevertheless, the treatment with these drugs is accompanied by several severe side effects, such as addictive behaviour and withdrawal syndromes [9]. Hence, there is an urgent need for new pharmaceutical ligands with a reduced side effect profile.
Another class of exogenous ligands for the GABAA receptors are pyrazoloquinolinones which showed interesting modulatory activities via a different binding site, namely the α + /β- interface [10]. However, these compounds still possess a promiscuous binding profile [2] and poor solubility in most polar solvents. Thus, they are investigated to become pharmacological tool compounds by variation of their substitution pattern [11,12,13,14,15,16].
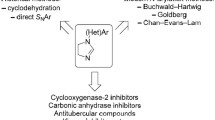
In line with the concept of pharmacophore modelling, the class of imidazoquinolines are a closely related chemotype to the pyrazoloquinolinones and differs mainly in the change of one hydrogen bond acceptor entity (Fig. 1). Therefore, they represent a promising class of compounds in terms of GABAA receptor activity [17]. Since synthetic routes towards this peculiar tricyclic scaffold are scarce [18,19,20], we wanted to investigate an alternative method to gain flexibility in synthesis. High functional group tolerance was the main prerequisite for a newly developed method. Moreover, we aimed to investigate the modulatory activity of the newly synthesized compounds at a GABAA receptor subtype.
Results and discussion
To allow the introduction of various substituents on ring D, a retrosynthetic analysis of lead structure I suggested two main building blocks: the diamino quinoline II and benzaldehyde or benzoic acid III (Scheme 1). This route allows late-stage modifications of substituents R1 on ring D via the use of different benzaldehyde or benzoic acid derivatives. However, the diamino quinoline II has to be synthesized for each substituent R2.

The alternative retrosynthetic cut b (Scheme 1) leads to IV and an appropriated substituted benzene V. Following this pathway, a direct arylation in position 2 can be envisioned taking advantage of transition metal catalysed C-H functionalization. In addition, in this case the corresponding building block IV would need to be prepared for each substituent R2 separately. Hence the difference lies in the final introduction of the aryl-moitey at C2. Additionally, it has to be kept in mind, that the C–H coupling at C2 might be in competition with N-arylation. To determine, which method would be the overall more efficient one, both approaches were investigated.
For both approaches, diaminoquinolines have to be prepared as a crucial intermediate. Synthetic routes towards these compounds have been published previously and were also established partially in our group [12].
The aniline starting materials 1a–1d determine the substitution pattern on ring A in the final molecule. Building up on our findings from the study on pyrazoloquinolinones we decided to use the unsubstituted aniline 1a, two halogenated anilines 1b and 1c and anisidine 1d to introduce a methoxy substituent [12]. The first step of the synthesis was the condensation of the anilines 1a–1d to the quinolones 3a–3d. This was done in a two-step process. In the first step, the anilines 1a–1d reacted with diethylmalonate in a Gould-Jacobs reaction [21] to form the corresponding enamines 2a–2d in excellent yields (90%—quantitative). Then, after a solvent exchange to a higher boiling solvent, the ring closure to quinolones 3a–3d was performed. In this step, high temperatures were required (> 150 °C) since intermediately aromaticity is lost. Not surprisingly, such high temperatures led to the formation of a significant amount of (unidentified) side products. Nevertheless, the desired carboxylated quinolones 3a–3d were obtained in acceptable yields (31–61%) and good purity, as they were hardly soluble in any organic solvent and could be easily purified by trituration. The decarboxylation of the ester group was achieved also in a two-step process: first, the ester was hydrolysed and after the carboxylic acid was isolated by precipitation, it was thermally decarboxylated to yield the quinolones 4a–4d, again by using Ph2O as a high-boiling solvent (Scheme 2).

Then, we introduced the nitro group in position 3 which was accomplished by standard nitration conditions using a mixture of nitric and acetic acid which gave the 3-nitroquinolones 5a–5d in good yields. Subsequently, chlorination was performed using POCl3, yielding the chlorinated nitroquinolines 6a–6d (Scheme 3).

The final steps towards the diamino quinolines now differed for halogen-bearing derivatives 6b and 6c, as milder reduction conditions had to be employed to avoid dehalogenation. Therefore, the amino group on position 4 was introduced directly using aq. ammonia in 1,4-dioxane, which gave the amino-nitro quinolines 7a–7c in quantitative yields. Reduction of the nitro group was carried out under modified Béchamp conditions [22] using iron and NH4Cl to give diamino quinolines 8a–8c (Scheme 4). If other reduction conditions, like palladium on charcoal under H2 atmosphere were employed, substantial dehalogenation was observed.

In cases where dehalogenation was not an issue, a route via azide 7d can be applied. Subsequent reduction using palladium on charcoal under a hydrogen atmosphere afforded the desired diamino quinoline 8d (Scheme 5).

The diamino quinolines 8a–8d now enabled the syntheses of the desired imidazoquinolines. Initially, compounds 9a–9d were prepared to test the direct arylation approach. Cyclization of 8a–8d with formic acid led to the target compounds in good to excellent yields (Scheme 6, 57–98%). Optimization of the direct arylation was carried out using 9a as substrate, as the simplest derivative of this series. First, we employed a protocol established by Bellina et al. for imidazoles, azoles, indoles, and benzimidazoles [23]. There, the C–H activation is carried out by a palladium-copper complex under ligand- and base-free conditions in DMF. When we applied the same conditions on 9a, the direct arylation towards the desired product 10a occurred, but only in 15% yield after a reaction time of 48 h (Table 1, entries 1 and 2). In this first experiment, we observed two main issues which decreased the formation of product. Most importantly, N-arylation of the nitrogen in position three indeed took place and led to significant side product formation. Furthermore, the solubility of the starting material 9a was low and precipitation occurred during the reaction.

Therefore, the catalytic system by displacement of palladium with a different transition metal species (Rh and Ni catalysts, see SI for details). In addition, the influence of different bases as well as ligands and additives was investigated (Table 1, entries 3 and 4, and SI). As this only had limited success, we built upon our initial successful transformation using Pd(OAc)2 and CuI in DMF and focused on finding conditions which prevent the precipitation of starting material. This might be caused by the formation of a copper salt, which has been reported previously for similar heterocyclic systems [24]. Therefore, we tried to reduce the amount of the copper source to a minimum which still allows the transformation. We found that a decreased amount of copper additive (0.6 equiv.) under prolonged reaction times (96 h) led to an increased isolated yield of 34% of the desired 10a (Table 1, entry 6). Further attempts to increase the yield by N-protection to suppress side product formation and to increase solubility failed (see SI).
Consequently, we switched to a more classical cyclization approach using either carboxylic acids or aldehydes as reaction partners. Benzaldehyde was used in combination with nitrophenol (as solvent and oxidant) to obtain the desired imidazoquinolines 10a and 10c [25]. The same conditions using now p-formylbenzonitrile could be applied to introduce a cyano group on ring D in the final product. This afforded the desired products 11a, 11b, and 11d in good yields (Scheme 6). However, the same methodology led only to the intermediary formed imine with p-methoxybenzaldehyde. Thus, we decided to use the more stable carboxylic acid under harsher conditions. Polyphosphoric acid, a strong acidic and hygroscopic reagent, which additionally binds the formed water allowed the synthesis of the methoxy substituted imidazoquinolines 12b and 12d.
Overall, we synthesized a library of 11 imidazoquinolines which we aimed to investigate their activity as allosteric modulators in GABAA receptors (Fig. 2).
The modulatory activity was investigated by testing the ligands in recombinant α1β3 GABAA receptors. The receptors were expressed in X. laevis oocytes and the compounds were screened at 1 µM and 10 µM at 3–5% (EC3-5) of the maximum GABA elicited current (see methods).
Compounds 11a, 11b, 11d (cyano substituted D ring) and 12b, 12d (methoxy substituted D ring) were tested to compare their activity with similarly substituted pyrazoloquinolinones [7, 8]. Interestingly, compound 11a displayed a significant modulatory activity (Fig. 3), while the other compounds were not sufficiently active to further characterize in this receptor subtype. Thus, compound 11a represents to the best of our knowledge the first imidazoquinolone which significantly modulates GABAA receptors independently of the presence of a benzodiazepine binding site.
Conclusion
We were able to establish a versatile synthetic route towards the class of imidazoquinolines, which already showed interesting activity on the tested GABAA receptor subtype. Compound 11a gave the most promising results and is the first example of a positive allosteric modulator of its class. The synthetic route was adapted in a way to allow the introduction of a large number of substituents, especially via the first shown CH activation of an imidazoquinoline towards 3-arylimidazoquinolines. Additionally, it is important to note that the imidazoquinoline products 10, 11, and 12 displayed increased solubility as compared to the corresponding pyrazoloquinolinones. Future research will be directed towards further improving the activity profile and establish the imidazoquinolones as subtype-selective GABAA receptor ligands.
Experimental
All starting materials and reagents were purchased from commercial sources and used without further purification. Reactions were monitored by TLC on silica gel 60 F254 plates. Normal-phase column chromatography was performed on silica gel 60 (230–400 mesh). NMR spectra were recorded at 297 K in the solvent indicated, with 200, 400, and 600 MHz instruments, respectively, employing standard software provided by the manufacturer. 1H NMR and 13C NMR spectra were referenced to tetramethylsilane (TMS, δ = 0) by calibration with the residual organic solvent signals [26]. Accurate mass analysis (2 ppm mass accuracy) was carried out from 10 to 100 mg/dm3 solutions via LC-TOFMS measurements using an autosampler, an HPLC system with binary pumps, degasser, and column thermostat and ESI-TOF mass spectrometer. Melting points were determined with a Büchi Melting Point B-545 apparatus with a heating rate of 1 °C/min (70% onset point and 10% clear point) or on a Kofler Block apparatus. All melting points were obtained without additional recrystallization directly after flash column chromatography (FCC) with light petroleum (LP) and EtOAc and subsequent drying in a high vacuum.
Two-electrode voltage clamp electrophysiology
All steps were performed as reported previously [14]. cDNA vectors were linearized, transcribed and purified to generate mRNAS, which were used for injecting Xenopus laevis oocytes. For the microinjection, the RNA of the α1β3 receptor combination was mixed at 1:1 ratio with a final concentration of 56 ng/mm3. Oocytes were obtained from commercial or local academic suppliers. Stage 5–6 oocytes with the follicle cell layer around them were roughly dissected with forceps, digested with collagenase (type IA, Sigma, NO, 1 mg/ml ND96 [96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES; pH 7.5)] at 18 °C shaking at 70 rpm for 20–40 min and gently defolliculated with the aid of a pipette and a platinum loop. For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Ca2+-containing NDE solution medium [96 mM NaCl, 5 mM HEPES–NaOH (pH 7.5), 2 mM KCl, 1 mM MgCl2‧6H2O, 1.8 mM CaCl2‧2H2O]. For current measurements, the oocytes were impaled with two microelectrodes (1–3 MΩ) filled with 2 M KCl. The oocytes were constantly washed by a flow of 6 cm3/min NDE that could be switched to NDE containing GABA and/or drugs. Drugs were diluted into NDE from DMSO-solutions resulting in a final concentration of 0.1% DMSO perfusing the oocytes. DMSO was used as blind control. Compounds were co-applied with GABA until a peak response was observed. All recordings were performed at room temperature at a holding potential of − 60 mV using a Dagan TEV-200A two-electrode voltage clamp (Dagan Corporation, Mineapolis, MN). Data were digitized, recorded and measured using an Axon Digidata 1550 low-noise data acquisition system (Axon Instruments, Union City, CA). Data acquisition was done using pCLAMP v.10.5 (Molecular Devices™, Sunnyvale, CA). Data were analysed using GraphPad Prism v.6. and plotted as bar diagrams/bar graphs. Data are given as mean ± SEM from at least three oocytes of two and more oocyte batches.
General procedure a: synthesis of malonates
According to a modified literature procedure [27], anilines 1a–1d (1.00 equiv.) and diethyl-2-ethoxymethylene malonate (1.00 equiv.) were dissolved in toluene (1.25 cm3/mmol). The reaction was heated to reflux, up to 22 h, until full conversion was observed by TLC (LP/EtOAc, 3:1). The reaction mixture was cooled to rt and the solvent was removed under reduced pressure. The oil residue obtained was lyophilized to give the desired products 2a–2d in adequate purity.
General procedure B: cyclization to quinolones
According to a modified literature procedure [28], malonates 2a–2d (1.00 equiv.) were dissolved in diphenyl ether (2 cm3/mmol), the atmosphere changed to argon and the reaction mixture was heated to reflux for one hour. The reaction time did not allow full conversion but disabled the formation of side products. The reaction mixture was cooled to rt and poured into LP to precipitate the desired quinolone which was collected by filtration and washed several times with a mixture of LP/EtOAc (1:1) to obtain the desired products 3a–3d.
General procedure C: decarboxylation
According to a modified literature procedure [29], quinolone carboxylates 3a–3d (1.00 equiv.) were suspended in 2 N NaOH solution (20 cm3/mmol). The reaction mixture was heated to reflux, up to 4 h, until full conversion was observed by TLC (CH2Cl2/MeOH, 9:1). The reaction mixture was cooled to rt and neutralized with 2 N HCl to precipitate the product. The product was collected by filtration, washed with 200 cm3 water and dried. Decarboxylation was performed in Ph2O (20 cm3/mmol). Carboxylic acid (1.00 equiv.) was suspended and heated to reflux (250 °C) up to 2 h until full conversion was observed by TLC (CH2Cl2/MeOH, 9:1). The reaction mixture was cooled to rt and poured into LP to precipitate the desired products 4a–4d which were collected by filtration, washed several times with LP and dried.
General procedure D: nitration
According to a modified literature protocol [30], quinolones 4a–4d (1.00 equiv.) were dissolved in AcOH (15 cm3/mmol) under heating. Concentrated HNO3 (2.2 equiv.) was diluted with AcOH (1:10) and added to the reaction mixture dropwise. The reaction mixture was heated to reflux for up to 4 h until the consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1). The reaction mixture became orange colored. After cooling to rt, the reaction was poured into water, whereupon the product precipitated. The precipitate was collected by filtration and washed with small amounts of EtOH and water and dried to yield the desired products 5a–5d.
General procedure E: chlorination of nitroquinolones
According to a modified literature protocol [31], nitroquinolones 5a–5d (1.00 equiv.) were dispersed in POCl3 (4.00 equiv.). The reaction mixture was heated to reflux for up to 4 h, until full consumption of the starting material was observed by TLC (LP/EtOAc, 3:1). The reaction mixture was poured on a small amount of ice whereupon precipitation occurred and neutralized with sat. aq. NaHCO3. The aqueous layer was extracted with CH2Cl2, washed with brine, dried over Na2SO4 and the solvent evaporated. The products 6a-6d were purified by FCC (gradient of 5%-15% EtOAc in LP).
General procedure F: synthesis of nitroquinolin-4-amines
According to a modified literature procedure [32], nitroquinolones 6a–6c (1.00 equiv.) were dissolved in 1,4-dioxane (10 cm3/mmol) and aq. NH4OH (25%, 10 cm3/mmol) was added. The reaction mixture was heated to reflux up to 1 h, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 19:1). After cooling to rt, a precipitate was formed. The solvent was evaporated and the precipitate was dissolved in EtOAc and extracted with water and brine, dried over Na2SO4 and evaporated to give the desired products 7a–7c.
Diethyl 2-[(phenylamino)methylene]malonate (2a)
[33] Applying general procedure A, using aniline (1a, 3.92 cm3, 43 mmol, 1.00 equiv.) and diethyl(ethoxymethylene)malonate (8.69 cm3, 43 mmol, 1.00 equiv.) were dissolved in toluene (54 cm3), giving 2a (11.4 g, 43 mmol, quant.). Light-pink waxy solid; m.p.: 47–49 °C (Ref. [33] 46–48 °C); 1H NMR (400 MHz, DMSO-d6): δ = 1.15–1.32 (m, 6H), 4.12 (q, J = 7.1 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 7.07–7.23 (m, 1H), 7.30–7.50 (m, 4H), 8.41 (d, J = 13.9 Hz, 1H), 10.70 (d, J = 13.9 Hz, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 14.2, 14.2, 59.4, 59.6, 93.1, 117.5, 124.6, 129.6, 139.4, 151.1, 164.9, 167.3 ppm. Literature reports NMR data solely in CDCl3.
Diethyl 2-[[(3-bromophenyl)amino]methylene]malonate (2b)
[15] Applying general procedure A, 3-bromoaniline (1b, 15.0 g, 87 mmol, 1.00 equiv.) and diethyl(ethoxymethylene)malonate (18.00 g, 87 mmol, 1.00 equiv.) were dissolved in toluene (109 cm3), giving 2b (26.0 g, 78 mmol, 90%). Yellow needles; m.p.: 71–73 °C (Ref. [15] 71–73 °C); NMR spectral data were found to be identical to the ones described in Ref. [15].
Diethyl 2-[(4-chlorophenylamino)methylene]malonate (2c)
[28] Applying general procedure A, 4-chloroaniline (1c, 5.00 g, 39.2 mmol, 1.00 equiv.) and diethyl(ethoxymethylene)malonate (7.92 cm3, 39.2 mmol, 1.00 equiv.) were dissolved in toluene (49 cm3), giving 2c (12.0 g, 39.2 mmol, quant.). Colourless waxy powder; m.p.: 79–81 °C (Ref. [28] 45–46 °C); NMR spectral data were found to be identical with the ones described in Ref. [28].
Diethyl 2-[[(4-methoxyphenyl)amino]methylene]malonate (2d)
[27] Applying general procedure A, 4-methoxyaniline (1d, 15.00 g, 122 mmol, 1.00 equiv.) and diethyl(ethoxymethylene)malonate (26.4 g, 122 mmol, 1.00 equiv.), giving 2d (35.7 g, 122 mmol, quant.). Yellow–brown solid; m.p.: 30–32 °C (Ref. [27] 38–40 °C); 1H NMR (400 MHz, CDCl3): δ = 1.31 (t, J = 7.1 Hz, 3H), 1.37 (t, J = 7.1 Hz, 3H), 3.79 (s, 3H), 4.23 (q, J = 7.1 Hz, 2H), 4.29 (q, J = 7.1 Hz, 2H), 6.85–6.94 (m, 2H), 7.02–7.11 (m, 2H), 8.43 (d, J = 13.8 Hz, 1H), 10.98 (d, J = 13.8 Hz, 1H) ppm; 13C NMR (101 MHz, CDCl3): δ = 14.3, 14.4, 55.6, 60.0, 60.2, 92.5, 115.0, 118.8, 132.8, 152.6, 157.2, 165.8, 169.2 ppm.
Ethyl 4-oxo-1,4-dihydroquinoline-3-carboxylate (3a)
[33] Applying general procedure B, diethyl 2-[(phenylamino)methylene]malonate (2a, 10.00 g, 38 mmol, 1.00 equiv.) was dissolved in Ph2O (76 cm3) to give 3a (4.3 g, 20 mmol, 52%). Light brown powder; m.p.: 270–271 °C (Ref. [33] 268–269 °C); 1H NMR (400 MHz, DMSO-d6): δ = 1.28 (t, J = 7.1 Hz, 3H), 4.21 (q, J = 7.1 Hz, 2H), 7.36–7.47 (m, 1H), 7.61 (dd, J = 8.4, 1.1 Hz, 1H), 7.65–7.77 (m, 1H), 8.15 (dd, J = 8.1, 1.4 Hz, 1H), 8.55 (s, 1H), 12.31 (br s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 14.3, 59.5, 109.8, 118.7, 124.6, 125.6, 127.2, 132.4 (d), 138.9, 144.8, 164.8, 173.4 ppm.
Ethyl 7-bromo-4-oxo-1,4-dihydroquinoline-3-carboxylate (3b)
[34] Applying general procedure B, diethyl 2-[[(3-bromophenyl)amino]methylene]malonate (2b, 26.90 g, 79 mmol, 1.00 equiv.) was dissolved in Ph2O (160 cm3) to give 3b (14.0 g, 15.1 mmol, 61%). Brown solid; m.p.: 297 °C (decomp.) [Ref. [34] 334–335 °C (decomp.)]; NMR spectral data were found to be identical to the ones described in Ref. [34].
Ethyl 6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylate (3c)
[35] Applying general procedure B, diethyl 2-[(4-chlorophenylamino)methylene]malonate (2c, 10.0 g, 33.6 mmol, 1.00 equiv.) was dissolved in Ph2O (67 cm3) to give 3c (3.80 g, 15.1 mmol, 45%). Colourless crystals; m.p.: > 300 °C (decomp.) [Ref. [35] > 200 °C (decomp.)]; 13C NMR (151 MHz, DMSO-d6): δ = 14.4, 59.8, 110.1, 121.3, 124.7, 128.4, 129.4, 132.6, 137.7, 145.3, 164.6, 172.3 ppm; 1H NMR spectral data were found to be identical with the ones described in Ref. [35].
Ethyl 6-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate (3d)
[36] Applying general procedure B, diethyl 2-[(4-methoxyphenylamino)methylene]malonate (2d, 14.30 g, 49 mmol, 1.00 equiv) was dissolved in Ph2O (100 cm3) to give 3d (3.8, 15.1 mmol, 31%). Brown solid; m.p.: 258–260 °C (Ref. [36] 274–276 °C); 13C NMR (151 MHz, DMSO-d6): δ = 14.4, 55.5, 59.5, 105.5, 108.7, 120.6, 122.2, 128.5, 133.4, 143.7, 156.6, 165.0, 172.9 ppm; 1H NMR spectral data were found to be identical with the ones described in Ref. [36].
Quinolin-4(1H)-one (4a)
[37] Applying general procedure C, ethyl 4-oxo-1,4-dihydroquinoline-3-carboxylate (3a, 2.62 g, 12.10 mmol, 1.00 equiv.) was saponified with 2 N NaOH (240 cm3) and decarboxylated with Ph2O (240 cm3) to give 4a (1.69 g, 11.6 mmol, quant.). Cream-coloured powder; m.p.: 203–204 °C; NMR spectral data were found to be identical to the ones described in Ref. [36].
7-Bromoquinolin-4(1H)-one (4b)
[38] Applying general procedure C, ethyl 7-bromo-4-oxo-1,4-dihydroquinoline-3-carboxylate (3b, 8.00 g, 27.0 mmol, 1.00 equiv.) was saponified with 2 N NaOH (540 cm3) and decarboxylated with Ph2O (540 cm3) to give 4b (7.20 g, 31.00 mmol, 92%). Colourless solid; m.p.: 345 °C (decomp.) (Ref. [38] 242–244 °C); NMR spectral data were found to be identical to the ones described in Ref. [38].
6-Chloroquinolin-4(1H)-one (4c)
[39] Applying general procedure C, ethyl 6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylate (3c, 3.70 g, 14.7 mmol, 1.00 equiv.) was saponified with 2 N NaOH (300 cm3) and decarboxylated with Ph2O (300 cm3) to give 4c (2.51 g, 14.00 mmol, 97%). Cream coloured crystals; m.p.: 270–272 °C (Ref. [39] 269–271 °C); 1H NMR (400 MHz, DMSO-d6): δ = 6.07 (d, J = 7.4 Hz, 1H), 7.59 (d, J = 8.9 Hz, 1H), 7.67 (dd, J = 8.9, 2.5 Hz, 1H), 7.95 (dd, J = 7.8, 3.2 Hz, 1H), 8.01 (d, J = 2.4 Hz, 1H), 11.99 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 108.9, 120.8, 123.9, 126.7, 127.8, 131.8, 138.7, 139.9, 175.6 ppm.
6-Methoxyquinolin-4(1H)-one (4d)
[40] Applying general procedure C, ethyl 6-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate (3d, 3.79 g, 15.0 mmol, 1.00 equiv.) was saponified with 2 N NaOH (300 cm3) and decarboxylated with Ph2O (300 cm3) to give 4d (2.23 g, 12.7 mmol, 85%). Colourless solid; m.p.: 250–251 °C (Ref. [40] 251–252 °C); 13C NMR (101 MHz, DMSO-d6): δ = 55.3, 104.2, 107.5, 120.0, 122.1, 126.8, 134.7, 138.4, 155.4, 176.2 ppm; 1H NMR spectral data were found to be identical with the ones described in Ref. [40].
3-Nitroquinolin-4(1H)-one (5a)
[31] Applying general procedure D, quinolin-4(1H)-one (4a, 2.30 g, 15.8 mmol, 1.00 equiv) was treated with conc. HNO3 (1.5 cm3) to give 5a (1.95 g, 10.30 mmol, 65%). Brown solid; m.p.: > 280 °C (decomp.) [Ref. [31] 357–358 °C (decomp.)]; 1H NMR (400 MHz, DMSO-d6): δ = 7.47–7.59 (m, 1H), 7.67–7.76 (m, 1H), 7.76–7.86 (m, 1H), 8.26 (dd, J = 8.1, 1.5 Hz, 1H), 9.19 (s, 1H), 13.00 (br s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 119.5(s), 125.8, 126.0, 128.1, 131.0, 133.2, 138.3, 142.4, 167.6 ppm.
7-Bromo-3-nitroquinolin-4(1H)-one (5b)
[41] Applying general procedure D, 7-bromoquinolin-4(1H)-one (4b, 5.00 g, 22.00 mmol, 1.00 equiv.) was treated with conc. HNO3 (2 cm3) to give 5b (4.20 g, 15.40 mmol, 70%). Yellow–brown solid; m.p.: 384.3 °C (decomp.); 1H NMR (200 MHz, DMSO-d6): δ = 7.68 (dd, J = 8.7, 1.9 Hz, 1H), 7.91 (d, J = 1.8 Hz, 1H), 8.16 (d, J = 8.7 Hz, 1H), 9.24 (s, 1H), 12.98 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 121.8, 126.6, 127.1, 128.2, 128.8, 131.4, 139.3, 143.0, 167.2 ppm.
6-Chloro-3-nitroquinolin-4(1H)-one (5c)
[31] Applying general procedure D, 6-chloroquinolin-4(1H)-one (4c, 2.20 g, 12.70 mmol, 1.00 equiv.) was treated with conc. HNO3 (1.1 cm3) to give 5c (1.60 g, 7.12 mmol, 56%). Cream-coloured solid; m.p.: > 300 °C (decomp.) (Ref. [31] > 300 °C (decomp.); 1H NMR (400 MHz, DMSO-d6): δ = 7.75 (d, J = 8.8 Hz, 1H), 7.85 (dd, J = 8.79, 2.45 Hz, 1H), 8.16 (d, J = 2.43 Hz, 1H), 9.24 (s, 1H), 13.15 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 121.9, 125, 129.4, 130.7, 131.1, 133.3, 137.1, 142.8, 166.6 ppm.
6-Methoxy-3-nitroquinolin-4(1H)-one (5d)
[42] Applying general procedure D, 6-methoxyquinolin-4(1H)-one (4d, 2.20 g, 13.0 mmol, 1.00 equiv) was treated with conc. HNO3 (1.2 cm3) to give 5d (2.10 g, 9.75 mmol, 75%). Yellow–brown solid; m.p.: 320 °C (decomp.) (Ref. [42] > 325 °C); 1H NMR (200 MHz, DMSO-d6): δ = 3.88 (s, 3H), 7.42 (dd, J = 9.0, 3.0 Hz, 1H), 7.59–7.75 (m, 2H), 9.12 (s, 1H), 12.99 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 55.6, 106.0, 121.2, 122.9, 129.5, 130.3, 132.6, 140.9, 157.4, 167.0 ppm.
4-Chloro-3-nitroquinoline (6a)
[31] Applying general procedure E, 3-nitroquinolin-4(1H)-one (5a, 1.80 g, 9.46 mmol, 1.00 equiv) was treated with POCl3 (3.5 cm3) to give 6a (1.83 g, 8.77 mmol, 93%). Pale yellow solid; m.p.: 119–120 °C (Ref. [31] 121–122 °C); 13C NMR (101 MHz, CDCl3): δ = 125.6, 126.1, 129.8, 130.4, 133.3, 136.7, 144.6, 149.3 ppm, signal of a quaternary carbon not detectable. 1H NMR spectral data were found to be identical to the ones described in Ref. [31].
7-Bromo-4-chloro-3-nitroquinolin (6b)
[45] Applying general procedure E, 7-bromo-3-nitroquinolin-4(1H)-one (5b, 0.70 g, 2.60 mmol, 1.00 equiv.) was treated with POCl3 (1.0 cm3) to give 6b (0.68 g, 2.37 mmol, 91%). Yellow–brown solid; m.p.: 150.9–151.1 °C; 1H NMR (400 MHz, CDCl3): δ = 7.90 (dd, J = 9.0, 1.9 Hz, 1H), 8.30 (d, J = 9.0 Hz, 1H), 8.41 (d, J = 1.9 Hz, 1H), 9.26 (s, 1H) ppm; 13C NMR (101 MHz, CDCl3): δ = 124.5, 127.3, 128.4, 132.8, 133.5, 137.0, 145.8, 149.7 ppm, signal of C3 not detectable.
4,6-Dichloro-3-nitroquinoline (6c)
[31] Applying general procedure E, 6-chloro-3-nitroquinolin-4(1H)-one (5c, 0.50 g, 2.23 mmol, 1.00 equiv.) was treated with POCl3 (0.8 cm3) to 6c (0.46 g, 1.89 mmol, 85%). Colorles solid; m.p.: 232–234 °C (Ref. [31] 165–167 °C); 1H NMR (400 MHz, CDCl3): δ = 7.88 (dd, J = 9.0, 2.3 Hz, 1H), 8.16 (dd, J = 9.0, 0.5 Hz, 1H,H8), 8.40 (dd, J = 2.3, 0.5 Hz, 1H), 9.23 (s, 1H) ppm; 13C NMR (101 MHz, CDCl3): δ = 124.9, 126.5, 132.0, 134.2, 135.6, 136.4, 141.8, 144.7, 147.7 ppm.
4-Chloro-6-methoxy-3-nitroquinoline (6d)
[31] Applying general procedure E, 6-methoxy-3-nitroquinolin-4(1H)-one (5d, 2.00 g, 9.10 mmol, 1.00 equiv.) was treated with POCl3 (3.4 cm3) to give 6d (0.90 g, 3.78 mmol, 42%). Yellow solid; m.p.: 113–114 °C (Ref. [31] 281–283 °C); 1H NMR (400 MHz, CDCl3): δ = 4.03 (s, 3H), 7.52–7.61 (m, 2H), 8.10 (dd, J = 8.7, 1.0 Hz, 1H), 9.09 (s, 1H) ppm; 13C NMR (151 MHz, CDCl3): δ = 56.1, 103.1, 126.3, 127.2, 131.9, 134.3, 141.9, 145.4, 160.5 ppm, signal of C3 not detectable.
3-Nitroquinolin-4-amine (7a)
[44] Applying general procedure F, 4-chloro-3-nitroquinoline (6a, 1.00 g, 4.75 mmol, 1.00 equiv.) was treated with aq. NH4OH (25%, 50 cm3) to give 7a (0.89 g, 4.70 mmol, quant.). Yellow solid; m.p.: 265–267 °C (Ref. [44] 265–266 °C); NMR spectral data were found to be identical to the ones described in Ref. [44].
7-Bromo-3-nitroquinolin-4-amine (7b, C9H6BrN3O2)
Appyling general procedure F, 7-bromo-4-chloro-3-nitroquinolin (6b, 0.679 g, 2.4 mmol, 1.00 equiv.) was treated with aq. NH4OH (25%, 24 cm3) to give 7b (0.64 g, 2.30 mmol, quant.). Yellow solid; m.p.: 245–246 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.76 (dd, J = 8.9, 2.1 Hz, 1H), 8.05 (d, J = 2.1 Hz, 1H), 8.51 (d, J = 9.0 Hz, 1H), 9.05 (s, 2H), 9.15 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 117.8, 123.4, 126.4, 126.4, 129.2, 131.4, 148.2, 148.6, 149.1 ppm; HRMS: m/z calc. for C9H7BrN3O2 ([M + H]+) 267.9716, found 267.9724.
6-Chloro-3-nitroquinolin-4-amine (7c, C9H6ClN3O2)
Applying general procedure F, 4-chloro-3-nitroquinoline (6c, 0.17 g, 0.70 mmol, 1.00 equiv.) was treated with aq. NH4OH (25%, 7 cm3) to give 7c (0.156 mg, 0.70 mmol, quant.). Yellow solid; m.p.: 295–297 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.85 (dd, J = 8.8, 2.0 Hz, 1H), 7.88 (dd, J = 8.9, 0.6 Hz, 1H), 8.76 (dd, J = 2.1, 0.7 Hz, 1H), 9.03 (s, 2H), 9.16 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 119.9, 123.5, 123.7, 131.1, 131.5, 132.9, 146.9, 147.4, 147.9 ppm; HRMS: m/z calc. for C9H7ClN3O2 ([M + H]+) 224.0221, found 224.0222.
3,4-Diaminoquinoline (8a)
[45] 3-Nitroquinolin-4-amine (7a, 850 mg, 4.50 mmol, 1.00 equiv.) was dissolved in methanol (250 cm3) and Pd/C (10% Pd, 85 mg) was added to the solution. Atmosphere was changed to H2 (balloon). The reaction was stopped after full consumption of starting material observed by TLC (CH2Cl2/MeOH, 19:1). The reaction mixture was filtered through a bed of Celite and solvent removed under reduced pressure to give 8a (0.74 g, 4.28 mmol, 95%). Brown solid; m.p.: 167–169 °C (Ref. [45] 171–173 °C); 1H NMR (400 MHz, DMSO-d6): δ = 4.72 (br s, 2H), 5.87 (br s, 2H), 7.21–7.38 (m, 2H), 7.61–7.69 (m, 1H), 7.95–8.02 (m, 1H), 8.20 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 118.5, 121.0, 123.4, 124.4, 125.1, 128.6, 134.0, 141.0, 142.7 ppm.
7-Bromoquinoline-3,4-diamine (8b, C9H8BrN3)
7-Bromo-3-nitroquinoline-4-amine (7b, 510 mg, 1.9 mmol, 1 equiv.) was suspended in EtOH/H2O (4:1, 20 cm3). Fe (1.05 g, 19 mmol, 10 equiv.) and NH4Cl (28.7 mg, 0.536 mmol, 1 equiv.) were then added to the mixture and the reaction was stirred and heated to reflux for up to 3 h until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1, + 3% Et3N). The reaction was then filtered through a bed of Celite. The filtrate was concentrated on SiO2 and the product 8b was obtained after purification by FCC using a gradient of MeOH in EtOAc + 3% Et3N (320 mg, 1.33 mmol 70%). Yellow–brown solid; m.p.: 126–128 °C; 1H NMR (400 MHz, DMSO-d6): δ = 4.84 (bs, 2H), 6.03 (s, 3H), 7.39 (dd, J = 9.0, 2.1 Hz, 1H), 7.81 (d, J = 2.1 Hz, 1H), 7.97 (d, J = 9.0 Hz, 1H), 8.19 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 117.2, 117.4, 123.4, 125.8, 126.2, 130.2, 134.3, 141.6, 143.4 ppm; HRMS: m/z calc. for C9H9BrN3 ([M + H]+) 237.9974, found 237.9983.
6-Chloroquinoline-3,4-diamine (8c)
[17] In a screw cap vial, 6-chloro-3-nitroquinoline-4-amine (7c, 120 mg, 0.536 mmol, 1 equiv.) was suspended in EtOH/H2O (4:1, 5 cm3). Fe (300 mg, 5.36 mmol, 10 equiv.) and NH4Cl (28.7 mg, 0.536 mmol, 1 equiv.) were then added to the mixture and the reaction was stirred and heated to reflux for up to 3 h until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1, + 3% Et3N). The reaction was then filtered through a bed of Celite. The filtrate was concentrated on SiO2 and the product 8c was obtained after purification by FCC using a gradient of MeOH in EtOAc + 3% NEt3 (84 mg, 0.43 mmol, 80%). Brown solid; m.p.: 215–217 °C (Ref. [17] 206–209 °C); 1H NMR (400 MHz, DMSO-d6): δ = 4.89 (s, 2H), 5.94 (s, 2H), 7.28 (dd, J = 8.9, 2.3 Hz, 1H), 7.65 (d, J = 8.9 Hz, 1H), 8.11 (d, J = 2.3 Hz, 1H), 8.20 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 119.0, 120.1, 125.1, 126.1, 128.3, 130.3, 133.8, 140.4, 140.5 ppm.
6-Methoxyquinoline-3,4-diamine (8d, C10H11N3O)
4-Chloro-6-methoxy-3-nitroquinoline (6d, 0.88 mg, 3.30 mmol, 1.00 equiv.) was dissolved in DMF (25 cm3). NaN3 (0.44 g, 6.60 mmol, 2.00 equiv.) was added and the reaction mixture was stirred for 20 min at rt. The solvent was removed in vacuo and the residue was dissolved in CH2Cl2. The organic layer was washed with water and brine and dried with MgSO4. The solvent was removed in vacuo and the product 7d was obtained in good purity and quantitative yield. Due to the instability of the azide group it was immediately used for the next reaction step without further purification. 4-Azido-6-methoxy-3-nitroquinoline (7d, 0.84 g, 3.40 mmol, 1.00 equiv.) was dissolved in MeOH and after the addition of Pd/C (10% Pd, 84 mg) the reaction was set under a hydrogen atmosphere (1 bar) and stirred at rt for 24 h. The reaction mixture was filtered through a bed of Celite. The product 8d was obtained after evaporation of the solvent (0.5 g, 2.54 mmol, 77%). Brown oil; 1H NMR (400 MHz, DMSO-d6): δ = 3.85 (s, 3H), 4.69 (s, 2H), 5.67 (s, 2H), 6.95 (dd, J = 9.1, 2.7 Hz, 1H), 7.30 (d, J = 2.8 Hz, 1H), 7.54 (d, J = 9.1 Hz, 1H), 8.06 (s, 1H) ppm; 13C NMR (101 MHz, CD3OD): δ = 56.0, 100.1, 119.8, 120.7, 125.4, 130.1, 137.9, 140.3, 140.5, 158.4 ppm; HRMS: m/z calc. for C10H12N3O ([M + H]+) 190.0982, found 190.0975.
Imidazo[4,5-c]quinoline (9a)
[45] 3,4-diaminoquinoline (8a, 1.47 g, 8.38 mmol, 1.00 equiv.) was suspended in trimethylorthoformate (170 cm3). The reaction mixture was heated under stirring until a clear solution was obtained. Then, formic acid (0.443 cm3, 11.8 mmol, 1.40 equiv.) was added carefully and a slight amount of precipitate was formed. Atmosphere was changed to argon, then reaction mixture was heated and stirred to reflux for 2 h, when full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1). The solvent was removed under reduced pressure and the solid obtained was washed several times with Et2O, sat. aq. NaHCO3, water and dried. The residual was purified by FCC (gradient of MeOH in CH2Cl2) to give the desired product 9a (0.966 g, 6.29 mmol, 75%). Cream coloured powder; m.p.: 285–288 °C (Ref. [45] 285–288 °C); 1H NMR (400 MHz, DMSO-d6): δ = 7.62–7.73 (m, 2H), 8.07–8.15 (m, 1H), 8.35–8.42 (m, 1H), 8.48 (s, 1H), 9.21 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 119.2, 121.5, 126.2, 126.8, 129.5, 133.4, 137.0, 142.3, 142.4, 143.5 ppm.
7-Bromo-5H-imidazo[4,5-c]quinoline (9b, C10H6BrN3)
7-Bromo-3,4-diaminoquinoline (8b, 20 mg, 0.08 mmol, 1 equiv.) was suspended in trimethylorthoformate (2 cm3). The reaction mixture was heated under stirring until a clear solution was obtained. Then, formic acid (4 mm3, 0.112 mmol, 1.4 equiv.) was added carefully and a slight amount of precipitate was formed. Atmosphere was changed to argon, then the reaction mixture was heated to reflux for 2 h when full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1). The solvent was removed under reduced pressure and the solid obtained was washed several times with Et2O, sat. aq. NaHCO3, water and dried in vacuo. The residual was purified by FCC (gradient of MeOH in CH2Cl2) to give the desired product 9b (14 mg, 0.05 mmol, 57%). Colourless solid; m.p.: 312–313 °C; 1H NMR (600 MHz, DMSO-d6, mixture of tautomers): δ = 7.85 (d, J = 8.3 Hz, 1H), 8.32 (d, J = 2.0 Hz, 1H), 8.35 (d, J = 8.7 Hz, 1H), 8.55 (s, 1H), 9.25 (s, 1H), 13.40 (s, 1H) ppm; 13C NMR (151 MHz, DMSO-d6, mixture of tautomers): δ = 119.8, 123.6, 126.9, 128.2, 129.4, 131.4, 142.8, 143.9, 144.2 ppm; HRMS: m/z calc. for C10H7BrN3 ([M + H]+) 247.9818, found 247.9819.
6-Chloro-3H-imidazo[4,5-c]quinoline (9c, C10H6ClN3)
6-Chloroquinoline-3,4-diamine (8c, 70 mg, 0.36 mmol, 1.00 equiv.) was suspended in trimethylorthoformate (7 cm3). The reaction mixture was heated under stirring until a clear solution was obtained. Formic acid (20 mm3, 0.5 mmol, 1.4 equiv.) was added carefully and a slight amount of precipitate was formed. Atmosphere was changed to argon and the reaction mixture was heated to reflux for 2 h until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 19:1). The solvent was removed under reduced pressure and the solid obtained was washed several times with Et2O, sat. aq. NaHCO3, water and dried in vacuo. The residual was purified by FCC (gradient of MeOH in CH2Cl2) to give the desired product 9c (74 mg, 0.343 mmol, 95%). Cream coloured solid; m.p.: > 300 °C (decomp.); 1H NMR (400 MHz, DMSO-d6): δ = 7.69 (dd, J = 9.0, 2.4 Hz, 1H), 8.13 (d, J = 8.9 Hz, 1H), 8.44 (d, J = 2.5 Hz, 1H), 8.54 (s, 1H), 9.24 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 120.5, 127.4, 130.8, 131.7,141.9 ppm; HRMS: m/z calc. for C10H7ClN3 ([M + H]+) 204.0329, found 204.0334.
8-Methoxy-5H-imidazo[4,5-c]quinoline (9d, C11H9N3O)
6-Methoxyquinoline-3,4-diamine (8d, 100 mg, 0.53 mmol, 1.00 equiv.) was suspended in trimethylorthoformate (11 cm3). The reaction mixture was heated under stirring until a clear solution was obtained. Then, formic acid (30 mm3, 0.74 mmol, 1.40 equiv.) was added carefully and slight amount of precipitate was formed. Atmosphere was changed to argon and the reaction mixture was heated to reflux for 2 h when full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 19:1). The solvent was removed under reduced pressure and the solid obtained was washed several times with Et2O, sat. aq. NaHCO3, water and dried in vacuo. The residual was purified by FCC (gradient of MeOH in CH2Cl2) to give the desired product 9d (50 mg, 0.25 mmol, 47%). Red solid; m.p.: 256–258 °C; 1H NMR (400 MHz, methanol-d4): δ = 3.99 (s, 3H), 7.32 (dd, J = 9.2, 2.8 Hz, 1H), 7.74 (s, 1H), 8.00 (d, J = 9.2 Hz, 1H), 8.41 (s, 1H), 8.97 (s, 1H) ppm; 13C NMR (101 MHz, methanol-d4): δ = 56.2, 101.4, 120.7, 131.1, 140.2, 143.6, 160.0 ppm, the signals of four carbons were not detectable; HRMS: m/z calc. for C11H10N3O ([M + H]+) 200.0818, found 200.0826.
2-Phenylimidazo[4,5-c]quinoline (10a)
[45] (a) Starting from 3,4-diaminoquinoline (8a, 40 mg, 0.23 mmol, 1.00 equiv.) and benzaldehyde (25 mm3, 0.24 mmol, 1.05 equiv.). After the addition of PhNO2 (2.5 cm3), the reaction mixture was heated to 150 °C overnight, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 9:1). Then, the mixture was concentrated on silica and the desired product 10a was obtained by FCC using a gradient of MeOH in CH2Cl2 from 5 to 20% (56 mg, 0.23 mmol, 98%).
(b) Via CH activation: In a screw cap vial, imidazo[4,5-c]quinoline (9a, 60 mg, 0.35 mmol, 1.00 equiv.), Pd(OAc)2 (4 mg, 0.018 mmol, 5 mol%), and CuI (40 mg, 0.212 mmol, 0.6 equiv.) were placed. Atmosphere was changed to argon and degassed DMF (2 cm3) was added. Subsequently, iodobenzene (80 mm3, 0.71 mmol, 2.00 equiv.) was added via syringe. The vial was placed in a heating block and stirred at 140 °C for 48 h. Then, the reaction mixture was filtered through Celite and concentrated in vacuo. The desired product was isolated with preparative HPLC to yield 10a (29 mg, 0.12 mmol, 34%). White-brown powder; m.p.: 283–285 °C (Ref. [45] 285–288 °C); 1H NMR (600 MHz, DMSO-d6): δ = 7.41 (t, J = 7.3 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.52–7.56 (m, 2H), 7.96–8.06 (m, 1H), 8.28–8.35 (m, 2H), 8.40–8.48 (m, 1H), 9.10 (s, 1H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 121.1, 122.7, 124.2, 125.4, 125.8, 127.1, 129.0, 129.7, 133.8, 138.2, 142.6, 142.9, 143.6, 156.5. ppm.
8-Chloro-2-phenyl-3H-imidazo[4,5-c]quinoline (10c, C16H10ClN3)
6-Chloroquinoline-3,4-diamine (9c, 70 mg, 0.36 mmol, 1.00 equiv.) and benzaldehyde (39 mm3, 0.38 mmol, 1.05 equiv.). After the addition of PhNO2 (5 cm3), the reaction mixture was heated to 150 °C overnight, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 19:1). The mixture was concentrated on silica and the desired product 10c was obtained by FCC using a gradient of MeOH in CH2Cl2 from 5 to 20% (71 mg, 0,25 mmol, 70%). Brown powder; m.p.: > 300 °C (decomp.); 1H NMR (400 MHz, DMSO-d6): δ = 7.54–7.59 (m, 1H), 7.60–7.66 (m, 2H), 7.70 (dd, J = 8.9, 2.4 Hz, 1H), 8.13 (d, J = 8.9 Hz, 1H), 8.23–8.32 (m, 2H), 8.50–8.64 (m, 1H), 9.24 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 120.8, 126.8, 127.4, 129.2, 129.4, 130.5, 130.8, 131.8, 142.0, 152.3 ppm; HRMS: m/z calc. for C16H11ClN3 ([M + H]+) 280.0642, found 280.0632.
4-(5H-Imidazo[4,5-c]quinolin-2-yl)benzonitrile (11a, C17H10N4)
3,4-Diaminoquinoline (8a, 80 mg, 0.5 mmol, 1.00 equiv.), 4-formylbenzonitrile (72 mg, 0.55 mmol, 1.1 equiv.) and ammonium acetate (39 mg, 0.5 mmol, 1.00 equiv.) were suspended in dry ethanol (5 cm3) and heated to reflux in an air atmosphere overnight until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 5:1), precipitation occurred and the solid was collected by filtration. The product 11a was obtained by washing with EtOH and water and drying in vacuo (58 mg, 0.22 mmol, 43%). M.p.: 388–391 °C; 1H NMR (600 MHz, DMSO-d6): δ = 7.71–7.75 (m, 2H), 8.03–8.19 (m, 3H), 8.37–8.62 (m, 3H), 9.28 (s, 1H), 14.13 (s, 1H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 112.2, 117.3, 118.6, 121.5, 122.0, 126.6, 127.2, 127.4, 127.7, 128.8, 129.8, 133.2, 133.8, 135.4, 137.5, 138.0, 143.8, 144.7, 149.6, 150.4 ppm; HRMS: m/z calc. for C17H11N4 ([M + H]+) 271.0985, found 271.0993.
4-(7-Bromo-5H-imidazo[4,5-c]quinolin-2-yl)benzonitrile (11b, C17H9BrN4)
7-Bromoquinoline-3,4-diamine (8b, 50 mg, 0.24 mmol, 1 equiv.) and 4-formylbenzonitrile (1.05 equiv.) were dissolved in PhNO2 (2.4 cm3), the reaction mixture was heated to 150 °C for 24 h, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 5:1). Then, the reaction mixture was concentrated on silica and product 11b were obtained by FCC, applying a gradient of MeOH in CH2Cl2 from 5 to 20% (52 mg, 0.17 mmol, 69%). Yellow solid; m.p.: 350 °C (decomp.); 1H NMR (400 MHz, DMSO-d6): δ = 7.82 (dd, J = 8.7, 2.0 Hz, 1H), 8.03 (d, J = 8.2 Hz, 2H), 8.26 (d, J = 1.9 Hz, 1H), 8.36 (d, J = 8.5 Hz, 3H), 9.22 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 112.7, 118.5, 119.0, 120.5, 124.3, 127.7, 129.9, 131.9, 133.5, 134.3, 139.2, 143.8, 144.8, 151.3 ppm; HRMS: m/z calc. for C17H10BrN4 ([M + H]+) 349.0083, found 349.0092.
4-(8-Methoxy-5H-imidazo[4,5-c]quinolin-2-yl)benzonitrile (11d, C18H12N4O)
6-Methoxyquinoline-3,4-diamine (8d, 45 mg, 0.24 mmol, 1.00 equiv.) and 4-formylbenzonitrile (33 mg, 0.25 mmol, 1.05 equiv.) were dissolved in PhNO2 (2.4 cm3) and the reaction mixture was heated to 150 °C for 24 h, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 5:1). Then, the reaction mixture was concentrated on silica and product 11d was obtained by FCC, applying a gradient of MeOH in CH2Cl2 from 5 to 20% (37 mg, 0.12 mmol, 52%). Red solid; m.p.: 143–145 °C; 1H NMR (600 MHz, DMSO-d6): δ = 3.98 (s, 3H), 7.32 (dd, J = 9.1, 2.9 Hz, 1H), 7.84 (d, J = 2.8 Hz, 1H), 8.02 (d, J = 9.1 Hz, 1H), 8.08 (d, J = 8.5 Hz, 2H), 8.41 (d, J = 8.2 Hz, 2H), 9.09 (s, 1H) 13.93 (s, 1H) ppm; 13C NMR (151 MHz, DMSO-d6, mixture of tautomers): δ = 55.6, 100.8, 112.2, 118.6, 118.8, 123.8, 127.2, 129.3, 131.1, 133.1, 133.89, 139.2, 149.8, 157.6 ppm; HRMS: m/z calc. for C18H13N4O ([M + H]+) 301.1084, found 301.1088.
7-Bromo-2-(4-methoxyphenyl)-5H-imidazo[4,5-c]quinoline (12b, C17H12BrN3O)
7-Bromoquinoline-3,4-diamine (8b, 50 mg, 0.29 mmol, 1 equiv.) and p-methoxybenzoic acid (53 mg, 0.35 mmol, 1.2 equiv.) were treated with PPA (0.5 cm3) was added and the reaction mixture was heated to 100 °C for 24 h, until full consumption of starting material was observed by TLC(CH2Cl2/MeOH, 5:1). The reaction mixture was then poured on H2O and adjusted to a pH of 9–10 with aq. NH4OH (25%), whereupon precipitation occurred. Precipitate was collected by filtration, washed with H2O and dried to give 12b (57 mg, 0.22 mmol, 77%). Brown solid; m.p.: 108–110 °C; 1H NMR (400 MHz, DMSO-d6, mixture of tautomers): δ = 3.85 (s, 3H), 7.15 (d, J = 8.8 Hz, 2H), 7.80 (m, 1H), 8.19 (m, 2H), 8.27 (d, J = 2.0 Hz, 1H), 8.40 (d, J = 8.8 Hz, 1H), 9.15 or 9.21 (s, 1H), 13.62 or 13.75 (s, 1H) ppm; 13C NMR (101 MHz, DMSO-d6, mixture of tautomers): δ = 55.4, 113.7, 114.5, 119.6, 121.9, 123.7, 128.3, 129.1, 131.4, 137.8, 138.3, 144.2, 145.1, 152.0, 153.1, 161.0 ppm; HRMS: m/z calc. for C17H13BrN3O ([M + H]+) 354.0236, found 354.0237.
8-Methoxy-2-(4-methoxyphenyl)-5H-imidazo[4,5-c]quinoline (12d, C18H15N3O2)
6-Methoxyquinoline-3,4-diamine (8d, 54 mg, 0.29 mmol, 1 equiv.) and p-methoxybenzoic acid (53 mg, 0.35 mmol, 1.2 equiv.) were treated with PPA (0.5 cm3) and the reaction mixture was heated to 100 °C for 24 h, until full consumption of starting material was observed by TLC (CH2Cl2/MeOH, 5:1). The reaction mixture was then poured on H2O and adjusted to a pH of 9–10 with aq. NH4OH (25%), whereupon precipitation occurred. Precipitate was collected by filtration, washed with H2O and dried to yield the desired product 12d (24 mg, 0.08 mmol, 28%). Red oil; 1H NMR (600 MHz, DMSO-d6): δ = 3.86 (s, 3H), 3.98 (s, 3H), 7.17 (d, J = 8.8 Hz, 2H), 7.29 (dd, J = 9.1, 2.9 Hz, 1H), 7.82–7.87 (m, 1H), 7.99 (d, J = 9.1 Hz, 1H), 8.21 (d, J = 8.3 Hz, 2H), 9.02 (s, 1H), 13.55 (s, 1H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 55.4, 55.6, 100.7, 114.5, 118.4, 122.2, 128.3, 131.1, 134.7, 137.7, 139.2, 141.4, 151.6, 157.3, 160.9 ppm; HRMS: m/z calc. for C18H16N3O2 ([M + H]+) 306.1237, found 306.1231.
References
Sieghart W (2015) Allosteric modulation of GABAA receptors via multiple drug-binding sites. In: Rudolph U (ed) Advances in pharmacology, vol 72. Academic Press, p 53
Iorio MT, Vogel FD, Koniuszewski F, Scholze P, Rehman S, Simeone X, Schnürch M, Mihovilovic MD, Ernst M (2020) Int J Mol Sci 21:334
Vega Alanis BA, Iorio MT, Silva LL, Bampali K, Ernst M, Schnürch M, Mihovilovic MD (2020) Molecules 25:999
Sigel E, Steinmann ME (2012) J Biol Chem 287:40224
Puthenkalam R, Hieckel M, Simeone X, Suwattanasophon C, Feldbauer RV, Ecker GF, Ernst M (2016) Front Mol Neurosci 9:44
Vinkers CH, Olivier B (2012) Adv Pharmacol Sci 2012:19
López-Muñoz F, Ucha-Udabe R, Alamo C (2005) Neuropsychiatr Dis Treat 1:329
Wang Q, Han Y, Xue H (1999) CNS Drug Rev 5:125
Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JRT, den Boer JA, Fineberg NA, Knapp M, Scott J, Wittchen HU (2005) J Psychopharm 19:567
Varagic Z, Wimmer L, Schnürch M, Mihovilovic MD, Huang S, Rallapalli S, Cook JM, Mirheydari P, Ecker GF, Sieghart W, Ernst M (2013) Br J Pharmacol 169:371
Varagic Z, Ramerstorfer J, Huang S, Rallapalli S, Sarto-Jackson I, Cook J, Sieghart W, Ernst M (2013) Br J Pharmacol 169:384
Treven M, Siebert DCB, Holzinger R, Bampali K, Fabjan J, Varagic Z, Wimmer L, Steudle F, Scholze P, Schnürch M, Mihovilovic MD, Ernst M (2018) Br J Pharmacol 175:419
Maldifassi MC, Baur R, Sigel E (2016) J Neurochem 138:722
Simeone X, Siebert DCB, Bampali K, Varagic Z, Treven M, Rehman S, Pyszkowski J, Holzinger R, Steudle F, Scholze P, Mihovilovic MD, Schnürch M, Ernst M (2017) Sci Rep 7:5674
Simeone X, Iorio MT, Siebert DCB, Rehman S, Schnürch M, Mihovilovic MD, Ernst M (2019) Biorg Med Chem 27:3167
Iorio MT, Rehman S, Bampali K, Stoeger B, Schnürch M, Ernst M, Mihovilovic MD (2019) Eur J Med Chem 180:340
Takada S, Sasatani T, Chomei N, Adachi M, Fujishita T, Eigyo M, Murata S, Kawasaki K, Matsushita A (1996) J Med Chem 39:2844
Thigulla Y, Akula M, Trivedi P, Ghosh B, Jha M, Bhattacharya A (2016) Org Biomol Chem 14:876
Larson P, Kucaba TA, Xiong Z, Olin M, Griffith TS, Ferguson DM (2017) ACS Med Chem Lett 8:1148
Guan ZR, Liu ZM, Ding MW (2018) Tetrahedron 74:7186
Gould RG, Jacobs WA (1939) J Am Chem Soc 61:2890
Wang Z (2001) Béchamp reduction. In: Comprehensive organic name reactions and reagents. Wiley, Hoboken
Bellina F, Calandri C, Cauteruccio S, Rossi R (2007) Tetrahedron 63:1970
Williams TJ, Bray JTW, Lake BRM, Willans CE, Rajabi NA, Ariafard A, Manzini C, Bellina F, Whitwood AC, Fairlamb IJS (2015) Organometallics 34:3497
Sharghi H, Asemani O, Khalifeh R (2008) Synth Commun 38:1128
Gottlieb HE, Kotlyar V, Nudelman A (1997) J Org Chem 62:7512
Kermack WO, Storey NE (1950) J Chem Soc 1950:607
Stern E, Muccioli GG, Millet R, Goossens JF, Farce A, Chavatte P, Poupaert JH, Lambert DM, Depreux P, Hénichart JP (2006) J Med Chem 49:70
Liebman KM, Burgess SJ, Gunsaru B, Kelly JX, Li Y, Morrill W, Liebman MC, Peyton DH (2020) Molecules 25:2251
Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, Gibson SJ, Imbertson LM, Jacobson JR, Knafla RT, Maye PV, Nikolaides N, Oneyemi FY, Parkhurst GJ, Pecore SE, Reiter MJ, Scribner LS, Testerman TL, Thompson NJ, Wagner TL, Weeks CE, Andre JD, Lagain D, Bastard Y, Lupu M (2005) J Med Chem 48:3481
Garcia-Echeverria C, Capraro HG, Furet P (2003) Preparation of 1H-imidazo[4,5-c]quinoline derivatives in the treatment of protein kinase dependent diseases. Chem Abstr 140:5048 (WO 03097641 A2, November 27, 2003)
Vernejoul F, Debin A, Drocourt D, Perouzel E, Tiraby G, Lioux T (2014) Preparation of conjugated TLR7 and/or TLR8 and TLR2 agonists. US Patent US 20140141033 A1, May 22, 2014; (2014) Chem Abstr 161:8697
Forezi L, Tolentino N, de Souza A, Castro H, Montenegro R, Dantas R, Oliveira M, Silva Floriano J, Barreto L, Burbano R, Abrahim-Vieira B, de Oliveira R, Ferreira V, Cunha A, Boechat F, de Souza M (2014) Molecules 19:6651
Chen YL, Zacharias J, Vince R, Geraghty R, Wang Z (2012) Bioorg Med Chem 20:4790
Stern E, Muccioli GG, Bosier B, Hamtiaux L, Millet R, Poupaert JH, Hénichart JP, Depreux P, Goossens JF, Lambert DM (2007) J Med Chem 50:5471
Medapi B, Suryadevara P, Renuka J, Sridevi JP, Yogeeswari P, Sriram D (2015) Eur J Med Chem 103:1
Huang J, Chen Y, King AO, Dilmeghani M, Larsen RD, Faul MM (2008) Org Lett 10:2609
Al-Awadi NA, Abdelhamida IA, Abdelhamidb IA, Al-Etaibib AM, Elngadia MH (2007) Synlett 2007:2205
Heindel ND, Kennewell PD, Fish VB (1969) J Heterocycl Chem 6:77
Hirano J, Hamase K, Zaitsu K (2006) Tetrahedron 62:10065
Larson PG, Ferguson DM (2021) Molbank 2021:M1305
Bachman GB, Welton DE, Jenkins GL, Christian JE (1947) J Am Chem Soc 69:365
Kshirsagar T, Lundquist G, Amos D, Dellaria J, Zimmermann B, Heppner P (2005) Preparation of imidazolyl hydroxylamine derivatives as antitumor and antiviral agents. Chem Abstr 143:26605 (WO 2005048945 A2, June 2, 2005)
Szpakiewicz B, Grzegozek M (2008) Can J Chem 86:682
van Galen PJM, Nissen P, van Wijngaarden I, Ijzerman AP, Soudijn W (1991) J Med Chem 34:1202
Acknowledgements
We are in deep sorrow as Daniele Catorci passed away in a tragic accident on June 27, 2020. His passion for organic chemistry and his cheerful temper will be missed in and outside the laboratory. We thank the Austrian Science Fund FWF for financial support to ME and MS (Grant P27446) and ME and MDM (Grant W1232).
Funding
Open access funding provided by Austrian Science Fund (FWF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Draskovits, M., Catorci, D., Wimmer, L. et al. Novel synthetic procedures for C2 substituted imidazoquinolines as ligands for the α/β-interface of the GABAA-receptor. Monatsh Chem 154, 1391–1404 (2023). https://doi.org/10.1007/s00706-022-02988-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02988-8