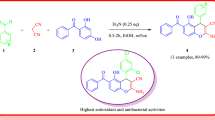
Abstract
An expedient, straightforward, and efficient one-pot three-component synthesis of biologically relevant indole-substituted 4H-chromenes has been developed by the reaction of various indoles, malononitrile, and salicylaldehydes using a catalytic amount of ascorbic acid in aqueous ethanol at room temperature. The significant features of the developed protocol are the clean reaction profile, operational simplicity, non-hazardous experimental conditions, no column chromatographic purification, use of an inexpensive, commercially available, and non-toxic metal-free catalyst, accomplishment of good to excellent yields, and large-scale synthesis.
Graphic abstract

Similar content being viewed by others
References
Elinson MN, Dorofeev AS, Miloserdov FM, Ilovaisky AI, Feducovich SK, Belyakov PA, Nikishin GI (2008) Adv Synth Catal 350:591
Kidwai M, Saxena S, Khan MK, Thukral SS (2005) Bioorg Med Chem Lett 15:4295
Ellis GP (1977) Chromenes, chromanones, and chromones. In: Weissberger A, Taylor EC (eds) The chemistry of heterocyclic compounds, chapter 2. Wiley, New York, p 11
Hafez EAA, Elnagdi MH, Elagamey AGA, El-Taweel FMAA (1987) Heterocycles 26:903
Bandini M, Eichholzer A (2009) Angew Chem Int Ed 48:9608
Das D, Pratihar S, Roy S (2013) Tetrahedron Lett 54:335
Mallick S, Mukhi P, Kumari P, Mahato KR, Verma SK, Das D (2019) Catal Lett 149:3501
Kausar N, Masum AA, Islam MM, Das AR (2017) Mol Divers 21:325
Kemnitzer W, Drewe J, Jiang S, Zhang H, Grundy CC, Labreque D, Bubenick M, Attardo G, Denis R, Lamothe S, Gourdeau H, Tseng B, Kasibhatla B, Cai SX (2008) J Med Chem 51:417
Shanthi G, Perumal PT (2007) Tetrahedron Lett 48:6785
Chen W, Cai Y, Fu X, Liu X, Lin L, Feng X (2011) Org Lett 13:4910
Singh N, Allam BK, Raghuvanshi DS, Singh KN (2013) Adv Synth Catal 355:1840
Rai P, Srivastava M, Yadav S, Singh J, Singh J (2015) Catal Lett 145:2020
Thakur A, Reddy PL, Tripathi M, Rawat DS (2015) New J Chem 39:6253
Gao Y, Du D-M (2013) Tetrahedron Asymmetry 24:1312
Rajesh UC, Wang J, Prescott S, Tsuzuki T, Rawat DS (2015) ACS Sustain Chem Eng 3:9
Brahmachari G, Nurjamal K (2016) Curr Green Chem 3:248
Rajesh UC, Kholiya R, Thakur A, Rawat DS (2015) Tetrahedron Lett 56:1790
Khalafi-Nezhad A, Nourisefat M, Panahi F (2015) Org Biomol Chem 13:7772
Bahuguna A, Choudhary P, Chhabra T, Krishnan V (2018) ACS Omega 3:12163
Shanthi G, Perumal PT, Rao U, Sehgal PK (2009) Indian J Chem 48B:1319
Li C-B, Li Y-W, Xu D-Z (2018) Synthesis 50:3708
Ganesan A, Kothandapani J, Subramaniapillai SG (2016) RSC Adv 6:20582
Schreiner PR (2003) Chem Soc Rev 32:289
Shaikh IR (2014) J Catal 2014:1
Oliveira VG, Cardoso MFC, Forezi LSM (2018) Catalysts 8:605
Das SK, Bhattacharjee P, Bora U (2018) ChemistrySelect 3:2131
Shaabani A, Khodkari V, Nazeri MT, Ghasemi S, Mohammadian R, Shaabani S (2019) J Iran Chem Soc 16:1793
Pariyar GC, Mitra B, Mukherjee S, Ghosh P (2020) ChemistrySelect 5:104
Paul D, Borah A, Khatua S, Chatterjee PN (2019) Asian J Org Chem 8:1870
Das D (2016) ChemistrySelect 1:1959
Acknowledgements
D.D. acknowledges the financial support (project ref. No YSS/2015/001425) from Science & Engineering Research Board (SERB), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, D. Ascorbic acid: an efficient organocatalyst for environmentally benign synthesis of indole-substituted 4H‑chromenes. Monatsh Chem 152, 987–991 (2021). https://doi.org/10.1007/s00706-021-02824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02824-5