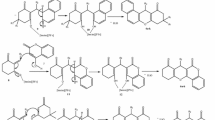
Abstract
In this study, haloepoxy, haloketones, halolactones, haloaldehydes, and haloalcohols which may have biological importance have been synthesized from ketene addition reactions. The structures of the molecules were illuminated by NMR, especially the COSY spectroscopy. In addition, FT-IR and GC–MS techniques were used. While doing the synthesis, we surprisingly encountered the quasi-Favorskii arrangement product. X-ray analysis was performed to determine the exact structure of this molecule. Furthermore, the antioxidant properties of some molecules were investigated and compared to standards (BHT, BHA, and α-tocopherol).
Graphic abstract




Similar content being viewed by others
References
Riordan JM, Kiely DE, DeLucas LJ, Einspahr HM, Bugg CE (1980) Carbohydr Res 82:303
Reynard E, Reymond JL, Vogel P (1991) Synlett 7:469
Takeda Y, Totani K, Matsuo I, Ito Y (2010) Bioorg Med Chem Lett 20:5357
McCasland GE, Furuta S, Bartuska V (1963) J Org Chem 28:2096
Cantekin S, Baran A, Çalışkan R, Balci M (2009) Carbohydr Res 344:426
Legler G (1977) Methods Enzymol 46:368
Salvucci ME (2000) Arch Insect Biochem Physiol 45:117
Trudel GC, Herscovics A, Holland PC (1988) Biochem Cell Biol 66:1119
Datema R, Romero PA, Legler G, Schwarz RT (1982) Proc Natl Acad Sci 79:6787
Syrpas M, Ruysbergh E, Stevens CV, Kimpe N, Mangelinckx S (2014) Beilstein J Org Chem 10:2539
Egorin MJ, Rosen DM, Benjamin SE, Callery PS, Sentz DL, Eiseman JL (1997) Cancer Chem Pharm 41:9
Brahmkshatriya PP, Brahmkshatriya PS (2013) Terpenes: chemistry, biological role, and therapeutic applications. In: Ramawat K, Mérillon JM (eds) Natural products. Springer, Berlin
Fuller RW, Cardellina JH, Jurek J, Scheuer PJ, Alvarado-Lindner B, McGuire M, Gray GN, Steiner JR, Clardy J, Menez E, Shoemaker RH, Newman DJ, Snader KM, Boyd MR (1994) J Med Chem 37:4407
Valeriote FA, Corbett TH, Baker LH (eds) (1994) Anticancer drug discovery and development: natural products and new molecular models. In: Proceedings of the second drug discovery and development symposium, Traverse City, Michigan, USA, June 27–29, 1991. Springer Science+Business Media
Wu YC, Luo SH, Mei WJ, Cao L, Wu HQ, Wang ZY (2017) E-J Med Chem 139:84
Gresley AL, Gudivaka V, Carrington S, Sinclair A, Brown JE (2016) Org Biomol Chem 14:3069
Yap TA, Workman P (2012) Annu Rev Pharmacol Toxicol 52:549
Marco-Contelles J, Molina MT, Anjum S (2004) Chem Rev 104:2857
Yılmaz Ö, Şimşek Kuş N, Küce P, Coral G, Çelik A, Gültekin MS (2015) Med Chem Res 24:2709
Held FE, Wei S, Eder K, Tsogoeva SB (2014) RSC Adv 4:32796
Theodore WH, Narang PK, Holmes MD, Reeves P, Nice FJ (1989) Ann Neurol 25:194
Tomson T, Almkvist O, Nilsson BY, Svensson JO, Bertilsson L (1990) Arch Neurol 47:888
Kuhnz W, Steldinger R, Nau H (1984) Dev Pharmacol Ther 7:61
Moschona F, Savvopoulou I, Tsitopoulou M, Tataraki D, Rassias G (2020) Catalysts 10:1117
Meninno S, Lattanzi A (2016) organocatalytic asymmetric reactions of epoxides: recent progress. Chemistry 22:3632–3642. https://doi.org/10.1002/chem.201504226
Nicolaou KC, Ray M, Finlay V, Ninkovic S, King NP, He Y, Li T, Sarabia F, Vourloumis D (1998) Chem Biol 5:365
Schobert R, Biersack B, Dietrich A, Effenberger K, Knauer S, Mueller T (2010) J Med Chem 53:6595
Kennedy TA, Lieber DC (1991) Chem Res Toxicol 4:290
Lieber DC, McClude TD (1996) Chem Res Toxicol 9:8
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Agric Food Chem 40:945
Sebille S, Gall D, Tullio P, Florence X, Lebrun P, Pirotte B (2006) J Med Chem 49:4690
Şimşek N, Arici C, McKee ML, Ülkü D, Balci M (2001) Struct Chem 12:305
Sengül ME, Simsek N, Balci M (2000) Eur J Org Chem 2000:1359
Kishali NH, Doğan D, Şahin E, Gunel A, Kara Y, Balci M (2011) Tetrahedron 67:1193
Yılmaz O, Bekfelavi EY, Kus NS, Tunc T, Sahin E (2017) Chem Pap 71:929
Bekfelavi EY, Küce Çevik P, Yılmaz Ö, Şimşek Kuş N, Coral G, Çelik (2018) Bioorg Med Chem Rep 1:6
Bekfelavi EY, Kuş NŞ (2019) J Mol Struct 1189:249
David J, Barreiros A, David (2004) J Pharm Biol 42:36
Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T (1989) Chem Pharm Bull 37:2016
Rigaku/MSC, Inc., 9009 New Trails Drive, TheWoodlands, TX 77381
Sheldrick GM (2008) Acta Cryst., A64, 112–122
Acknowledgements
This academic work was linguistically supported by the Mersin Technology Transfer Office Academic Writing Center of Mersin University [Grant no. 2019-3-AP4-3774 and 2015-TP3-1236]. We are grateful to Professor Hamdullah Kılıç for their help during this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bekfelavi, E.Y., Yılmaz, Ö., Şahin, E. et al. Novel halo-molecules; synthesis, structure elucidation, mechanism, and antioxidant activity. Monatsh Chem 152, 295–304 (2021). https://doi.org/10.1007/s00706-021-02746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02746-2