Abstract
This paper presents a novel, automatic, simple approach to stop-flow photometric determination of Fe(II) in wastewater and wine samples using a multi-pumping flow system with a direct-injection detector. The basis for the determination was the reaction of Fe(II) with 1,10-phenanthroline, which was carried out in the reaction chamber of the direct-injection detector. The research included a selection of appropriate parameters of the proposed analytical procedure and method validation. Under optimized conditions, linear calibration curves were obtained in two concentration ranges of Fe(II) 0.07–1.00 and 1.00–7.00 mg/dm3, with the quantification limit of 0.07 mg/dm3. The procedure was validated by studying the accuracy (8.2%, RE) and precision (9.6 and 14.8%, RSD, for higher and lower concentration range, respectively). The proposed method was successfully employed in Fe(II) determination in spiked wastewater and wine samples with recovery of 95.8–104.5%. Using the procedure, time of a single analysis (for three independently measured signals) was about 300 s and sample and reagent consumptions were 240 and 60 mm3, respectively.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of flow analysis techniques has created new possibilities for mechanization or automation of analytical procedures. The use of flow systems allows for mechanical insertion and transportation of solutions, as well as detection in continuous flow conditions. The essence of flow techniques is, however, the possibility of on-line preparation of a sample for analysis through dilution, conducting chemical reactions, preconcentration, or separation of an analyte from the sample matrix using various techniques, like extraction, dialysis, and precipitation. Application of flow techniques often helps improve the precision of analytical results. Moreover, it is consistent with the rules of green analytical chemistry by, among others, reducing the consumption of reagents, and, consequently, the amount of waste produced, increasing the sample throughput, reducing the cost of analyses, as well as limiting the operator’s contact with toxic reagents. Basic flow analysis techniques include continuous flow analysis (CFA), flow injection analysis (FIA), and sequential injection analysis (SIA). Their numerous modifications were also developed [1, 2].
Spectrophotometric determination of Fe(II) is often based on well-recognized reaction with 1,10-phenanthroline [3,4,5,6,7,8,9,10] or ferrozine [11,12,13,14,15,16,17,18]. Methods based on the formation of Fe(II) complexes with 2,2′-dipyridylketone picolinoylhydrazone [19] or 2-(5-bromo-2-pyridylazo)-5-(diethylamino)phenol [20, 21] have been also proposed to determine Fe(II) [19, 20] or total iron [21]. Fe(II) was also determined after separating Fe species using ion chromatography [3, 22] or liquid/liquid and column solid-phase extraction [23] with flame atomic absorption spectrometry detection, or HPLC method with electrochemical detection [24]. The approaches were applied to the determination of Fe(II) (also to iron speciation analysis or total iron determination) in water [3, 6,7,8,9, 12, 14, 17, 18], sea water [11, 12, 14, 16, 18], wastewater [13], wine [18,19,20,21,22,23,24], acid leachable fractions of sediment and soil [4], and pharmaceutical product [5] samples. Among them several flow-based systems, like flow injection [3,4,5,6,7, 11,12,13], multisyringe flow injection [21], sequential injection [8], and lab-in-syringe [9], have been reported.
One of the instrumental solutions used in flow techniques involves multi-commutation (MC) [25] and multi-pumping (MP) [26] systems (with electromagnetic valves and/or pumps) coupled with an original direct-injection detector (DID) [27]. In the DID, the sample and the reagent are simultaneously injected (in a countercurrent to ensure proper mixing of solutions) into a reaction chamber, which also serves as a detection chamber. The internal volume of the developed detection–reaction chamber (made of PTFE) was about 60 mm3 (i.d. 2 mm, length 20 mm), and two diodes played the roles of the light source and the real detector [27]. The system was adapted to determine Fe(III) in groundwater using the thiocyanate method [28]. The developed approach allowed for determination of Fe(III) in the concentration range of 0.15–10 mg/dm3 with a relative error (RE) not exceeding 1.5%. The method allowed 180 signals per hour to be recorded. 20 mm3 of both the sample and the reagent was consumed per repetition. The approach was also adapted to chemiluminescent determination of Fe(III) basing on the Fenton reaction with a reagent containing luminol and H2O2, enabling determination of Fe(III) with RE of 2% [29]. The linear range of the method was in the range of 0.5–10 mg/dm3, while the detection limit was 0.025 mg/dm3. During the analysis, it was possible to record 144 signals per hour.
In this work, the MP DID system was adapted for the first time to determine Fe (II) using 1,10-phenanthroline. Parameters of the proposed analytical procedure were selected and the method was validated. The usefulness of the developed approach was examined by determination of Fe(II) in wastewater and wine samples.
Results and discussion
Flow system and procedure
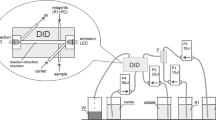
The solenoid micropump-based flow system with a direct-injection (DID) photometric detector equipped with paired emitter-detector diodes (PEDD) applied in determination of Fe(II) has been schematically shown in Fig. 1. It was composed of three solenoid micropumps (P1–P3) and PEDD direct-injection detector. P1 and P2 with nominal volume of 20 mm3 per pulse were used for injecting the sample and 1,10-phenanthroline, respectively, whereas P3 with nominal volume of 40 mm3 per pulse was used for cleaning DID with water. A special electronic adapter was used to control all the components of the flow system using computer software.
When cleaning, the chamber of DID was filled up with the water stream (400 mm3) using pump P3. Baseline was established in stop-flow mode. Next, 80 mm3 of the sample was injected using pump P1 and then (after 7 s) 20 mm3 of 1,10-phenanthroline was also injected. At that moment, the chemical reaction started and the measurement of absorbance was performed in stop-flow conditions after 70 s.
Optimization of experimental parameters
The studies were focused on searching for appropriate experimental conditions that would result in obtaining the highest analytical signal with the best precision. These studies included selecting concentration of 1,10-phenanthroline, time of reaction of the sample with 1,10-phenanthroline, and volume of sample, based on single-parameter optimization approach. The volume of 1,10-phenanthroline was established for the minimum value possible to inject with the pump used in the system (20 mm3).
Concentration of 1,10-phenanthroline was studied in the range of 0.2–2.0% by its reaction with Fe(II) at a concentration of 10 mg/dm3. In the case of 1,10-phenanthroline in concentrations of 1.0 and 2.0%, the precipitate was formed in the solutions after few hours of their preparation that prevented their use for further measurements. The value of 0.5% was selected as the signal (of the formed compound) reached a plateau much faster for this concentration of 1,10-phenanthroline than it did for 0.2% (Fig. 2).
Time of reaction of sample with 1,10-phenanthroline was also assessed. With this aim, Fe(II) solution (10 mg/dm3) was introduced into the reaction-detection chamber of DID, followed by the solution of 1,10-phenanthroline (0.5%). The signal was registered in stopped-flow mode for 200 s. It was noticed that 70 s after the reagent injection, the signal practically did not change (Fig. 2). Precision of signals studied for a standard solution containing Fe(II) at concentration 0.4 mg/dm3 and based on signals registered six times was 9.8%. The choice of time of 70 s allowed obtain results with acceptable precision in a relatively short time.
To study the sample volume, Fe(II) solutions of various volumes between 20 and 80 mm3 were tested (Fig. 3). It can be noted that when the volume of sample injected into the DID chamber increased, both improved sensitivity and linearity of the method were observed (see Fig. 3). For volumes (20 and 40 mm3) smaller than the DID chamber capacity (60 mm3), the sample (after injection) washed part of the water (filling the DID chamber) and it was mixed with the rest of the water. For a volume of 80 mm3, the sample solution washed and replaced water to much more extent (the range of dilution with water was limited); hence, the better sensitivity and linearity of calibration graph were obtained. For this reason, and because of the shorter analysis time and less sample consumption, the introduction of larger volumes was not tested, and a volume of 80 mm3 was used in further studies. Using the developed procedure, time of a single analysis (for three repetitions) was about 300 s and the sample and reagent consumptions were 240 and 60 mm3, respectively.
Figures of merit of developed approach
Under the conditions defined above, the main figures of merit of the developed method were studied. The linearity of the calibration graph responsible for Fe(II) determination was studied in concentration range from 0.05 to 8.00 mg/dm3. A signal for blank was measured and its value was subtracted from the standard signals. It was found that, based on the received signals, two linear calibration graphs (R = 0.999) could be prepared for the lower and the higher analyte concentration ranges, from 0.07 to 1.00 mg/dm3 (based on 10 standard solutions, A = 0.051c + 0.013; A—absorbance, c—Fe(II) concentration) and from 1.00 to 7.00 mg/dm3 (based on eight standard solutions, A = 0.043c + 0.022), respectively.
The limits of detection (LOD) and quantification (LOQ) were estimated with the use of standard solution containing 0.10 mg/dm3 of Fe(II). They were calculated as concentrations corresponding to the ratio of the signal standard deviation to the slope value and multiplied by 3 and 10, respectively. The values of 0.02 and 0.07 mg/dm3 were obtained for LOD and LOQ, respectively.
Accuracy of the approach was assessed by carrying out determination of Fe(II) in synthetic samples. A set of synthetic samples containing Fe(II) at different concentrations was prepared and analyzed. Each sample was analyzed three times using the system presented in Fig. 1. The analytical results with calculated values of relative standard deviation (RSD, %) and relative error (RE, %) are presented in Table 1.
Generally, it can be concluded that using the developed procedure, Fe(II) was determined with acceptable accuracy, lower than 8.2% (RE). Similarly, it can be assumed that results were obtained with acceptable precision lower than 10% (RSD). However, it should be noted that worse values of RSD were obtained for the results in lower concentration range (the worst, 14.8%, for the lowest Fe(II) concentration, close to the quantification limit) than in higher range (8.7%). Figures of merit of the developed procedure are summarized in Table 2.
Determination of Fe(II) in wastewater and wine samples
In order to verify the applicability of the developed approach for the determination of Fe(II) in samples of different matrix, quantification of Fe(II) was carried out for wastewater certified reference material (CRM) and wine samples, as well as for samples spiked with the analyte.
Iron content is an important parameter for controlling the quality and stability of wine. The level of its concentration in wine depends, among others, on the type of grapes, soil characteristics, environmental conditions, and contamination during the manufacturing process [19, 20]. In airtight conditions, iron exists mostly as Fe(II). However, after aerating wine, dissolved oxygen oxidizes Fe(II) to Fe(III), which is responsible for the precipitation of coloring matter (blue casse) and for the cloudiness in white wines (white casse) [20]. In the cited publications, the Fe(II) concentration varied in the range from 0.16 to 4.89 in white wine [18,19,20, 22, 23], from 2.49 to 2.55 in rose wine [19], and from 0.79 to 9.32 in red wine [19, 20, 22] samples.
White and rose wine samples were analyzed. For wastewater and wine samples, the interpolative and extrapolative calibration methods were used, respectively. Six calibration standards or three standard additions were used to prepare calibration graphs, respectively. Samples were analyzed three times and the mean values with confidence intervals (with significance level α = 0.05) were calculated. Results are presented in Table 3. To evaluate the agreement between the obtained and expected concentrations (determined plus spiked concentrations), the values were compared with each other using the Student’s t test (α = 0.05). It was confirmed that the determined concentrations were consistent with the expected values. The recovery of Fe(II) in wastewater sample did not exceed 104.5%, whereas in wine samples it ranged from 95.8 to 102.7%. The low Fe(II) content in the wine samples was due to the fact that the wine was analyzed several weeks after opening.
It can be concluded that the obtained results confirmed the analytical usefulness of the developed approach and the possibility of its application to determine Fe(II) in wastewater and white and rose wine samples. It should be also noted that the developed flow system has a chance to be applied to determine Fe(II) in water samples.
Comparing the analytical features of the proposed approach with those of previously developed flow-based methods (Table 4), it can be concluded that linear ranges of the methods are similar, although lower detection limits were achieved in some of the methods described earlier. The disadvantage of the developed approach is the precision, acceptable, but lower than for other developed methods (usually below 5%, RSD). On the other hand, the advantages of the developed system are low reagent consumption (comparable to the consumption using the SIA system), time of analysis (much shorter than using SIA systems), simplicity, and low costs of the system. Most of the reported systems have been developed for the determination of Fe(II) and Fe(III) ions. It should be noted that the proposed system can be also adapted to iron speciation analysis, to Fe(III) determination using the approaches developed earlier [28, 29] or after reduction of Fe(III) to Fe(II), or as a part of multi-detection system to determine Fe(II) and total iron using AAS or ICP OES method. The further research is going to be conducted in this direction.
Conclusions
A novel, automatic, simple, and inexpensive method has been proposed for Fe(II) determination. The approach is based on the use of a multi-pumping photometric flow system with a direct-injection detector and on the reaction of Fe(II) with 1,10-phenanthroline carried out in the reaction chamber of the direct-injection detector. The method was validated with acceptable accuracy (8.2%, RE) and precision (9.6 and 14.8%, RSD, for higher and lower concentration ranges, respectively). Good recovery values (95.8–104.5%) proved that developed method can be used for Fe(II) determination in wastewater and wine samples. Using the procedure, a sample can be analyzed in short time (about 300 s) with low sample and reagent consumptions 240 and 60 mm3, respectively.
It can be also noted that the developed flow system has a chance to be applied to determine Fe(II) in water samples. It could be also adapted to iron speciation analysis.
Experimental
Reagents and solutions
A stock standard solution of Fe(II) was prepared daily, by dissolving 0.176 g of (NH4)2Fe(SO4)2·6H2O (Chempur, Poland) in 16 cm3 of H2SO4 (1 mol/dm3) and bringing it up to 25.0 cm3 with water. The stock solutions were diluted appropriately with water to form intermediate or working standard solutions and synthetic samples. The solutions of 1,10-phenanthroline (0.2, 0.5, 1.0, and 2.0% (m/V)) were prepared by dissolving the appropriate amount of 1,10-phenanthroline monohydrate (Lach-Ner, Czech Republic) in HCl (0.1 mol/dm3) in 100.0 cm3 volumetric flask. The hydrochloric and sulfuric acids were prepared by appropriate dilution of 37% HCl (Merck, Germany) and 98% H2SO4 (Merck, Germany), respectively, with water.
Reagents of analytical grade were used. Substance for preparation of stock standard solution was weighed to the nearest 0.0001 g on analytical balance (RADWAG, Poland). Deionized water (specific conductance under 0.05 µS/cm) obtained from HLP5sp system (Hydrolab, Poland) was used throughout this study.
Certified reference material (CRM) of wastewater (EnviroMAT Wastewater, High (EU-H-3) Lot Number: SC8301825 (SCP SCIENCE, USA)) was diluted in accordance with the instructions with water. Samples of wine obtained at a local shop were put into ultrasonic bath (Sonic 3, Polsonic, Poland) for 10 min before the analysis.
Instrumentation
Three solenoid micropumps (ColeParmer, USA) with nominal volume of 20 and 40 mm3 per pulse were used. The direct-injection (DID) photometric detector based on paired emitter-detector diodes (PEDD) (KSP, Poland) was made from one block of PTFE. The volume of the reaction–detection chamber was about 60 mm3, 20 mm in length and 2 mm in diameter. Two paired LEDs (Huey Jann Electronic, Taiwan) were placed, one on each end. One LED was used as the light source and other as the light detector. The maximum of the emission spectrum of the LED used as a light source matched the maximum of absorption of the Fe(II)–1,10-phenanthroline complex (λmax = 512 nm). As an emission and detection diode, a green LED with λmax = 525 nm and a yellow-green LED with λmax = 570 nm (which can detect light of wavelength from 470 to 570 nm) were selected, respectively.
PEDD DID was described in detail previously [27]. PTFE tubing i.d. 0.8 mm (IDEX Health and Science, USA) was used as tubes. A special electronic adapter (KSP, Poland) was used to control all the components of the flow system and to register the signal using computer software.
References
Melchert WR, Reis BF, Rocha FRP (2012) Anal Chim Acta 714:8
Trojanowicz M (ed) (2008) Advances in flow analysis. Wiley, Weinheim
Chen S, Li N, Zhang X, Yang D, Jiang H (2015) Spectrochim Acta Part A Mol Biomol Spectrosc 138:375
Kozak L, Niedzielski P, Wachowiak K (2013) Microchem J 110:54
Oliveira AF, Nóbrega JA, Fatibello-Filho O (1999) Talanta 49:505
Kozak J, Gutowski J, Kozak M, Wieczorek M, Kościelniak P (2010) Anal Chim Acta 668:8
Kozak J, Jodłowska N, Kozak M, Kościelniak P (2011) Anal Chim Acta 702:213
Kozak J, Paluch J, Węgrzecka A, Kozak M, Wieczorek M, Kochana J, Kościelniak P (2016) Talanta 148:626
Paluch J, Kozak J, Wieczorek M, Kozak M, Kochana J, Widurek K, Konieczna M, Kościelniak P (2017) Talanta 171:275
Mohamed AA, Shalaby AA (2019) Food Chem 274:360
Sarradin PM, Le Bris N, Le Gall C, Rodier P (2005) Talanta 66:1131
Giokas DL, Paleologos EK, Karayannis MI (2002) Anal Bioanal Chem 373:237
Pascual-Reguera MI, Ortega-Carmona I, Molina-Dı́az A (1997) Talanta 44:1793
Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Van Cappellen P (2000) Appl Geochem 15:785
Thompsen JC, Mottola HA (1984) Anal Chem 56:755
King DW, Lin J, Kester DR (1991) Anal Chim Acta 247:125
Waterbury RD, Yao W, Byrne RH (1997) Anal Chim Acta 357:99
Capitán-Vallvey LF, Arroyo E, Berenguer C, Fernández-Ramos M, Avidad DR (2001) Fresenius J Anal Chem 369:139
López-López JA, Albendín G, Arufe MI, Mánuel-Vez MP (2015) J Agric Food Chem 63:4545
Ferreira SLC, Ferreira HS, de Jesus RM, Santos JVS, Brandao GC, Souza AS (2007) Anal Chim Acta 602:89
Phansi P, Kanchana K, Ferreira SLC, Cerda V (2019) Food Chem 277:261
Ajlec R, Stupar J (1989) Analyst 114:137
Tasev K, Karadjova I, Arpadjan S, Cvetkovic J, Stafilov T (2006) Food Control 17:484
Weber G (1991) Fresenius J Anal Chem 340:161
Rocha RP, Reis BF, Zagatto EAG, Lima JLFC, Lapa RAS, Santos JLM (2002) Anal Chim Acta 468:119
Lapa RAS, Lima JLSC, Reis BF, Santos JLM, Zagatto EAG (2002) Anal Chim Acta 466:125
Koronkiewicz S, Kalinowski S (2011) Talanta 86:436
Koronkiewicz S, Kalinowski S (2012) Talanta 96:68
Koronkiewicz S, Kalinowski S (2015) Talanta 133:112
Acknowledgements
The authors are grateful for financial support received from the National Science Centre, Poland (Opus, 2017–2020, Grant No. 2016/23/B/ST4/00789).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paluch, J., Kościelniak, P., Molęda, I. et al. Novel approach to determination of Fe(II) using a flow system with direct-injection detector. Monatsh Chem 151, 1305–1310 (2020). https://doi.org/10.1007/s00706-020-02649-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02649-8