Abstract
The poly-dentate ligand poly(4-vinylpyridine) (P4VP, Mw ≈ 60,000 g mol−1) reacts with (Me2S)AuCl or ZnCl2 yielding white polymers with empirical formula (PVP)(AuCl)0.4 and (PVP)(ZnCl2)0.7. Upon excitation with UV light, both metal-containing materials feature luminescence. Emissions of (P4VP)–AuCl are based on aurophilic interactions whereas for (P4VP)–ZnCl2, an excitation wavelength-dependent luminescence could be observed. The latter behavior can be explained by the formation of aggregated species with different excitation energies. For comparison, the model compound (Etpy)AuCl (Etpy = 4-ethylpyridine) is prepared and structurally characterized. The solid-state structure reveals that no aurophilic or π–π interactions are present. The compound does not show photoluminescence. The solid-state structure of the disproportionation product (Etpy)AuCl3 has been determined as well.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last years, research on transition metal-containing polymers increased significantly. Incorporation of transition metal ions into polymeric materials leads to a change of mechanic, electronic, optic, or magnetic properties [1,2,3,4,5,6,7,8,9]. Different strategies have been exploited to prepare metal-containing polymers leading to different types of polymers. In principle, the metal ions can be part of the polymeric backbone or part of the side groups. In the first case, the polymer is formed in a reaction not unlike a classical step-growth polymerization which leads to a material which most obvious term would be “coordination polymer” or in branched versions “coordination networks”. However, these terms are already occupied for material where metal ions and ligands form a polymer or network in solid state but dissociate into its components upon dissolution—if the material is soluble at all what actually rarely is the case. On the contrary, metal-containing polymers do not disassemble in solution but stay intact in a macromolecular form. According to a convention, these polymers are referred to as type II polymers [4, 5].
Polymers with functional groups which are capable to coordinate to a metal ion form metal-containing polymers of type I [4, 5]. The variety of these groups is as large as it is the case for classical non-polymeric ligands. Although it is in principle also possible to polymerize monomeric complexes bearing a polymerizable group, maybe the easiest way to synthesize such material is the use of commercially available polymers which contain a ligand as side group. For example, a huge number of different types of polymers, e.g., polyolefins and polyacrylates, which are functionalized with carboxylates, amines, alcohols, etc. are offered by several suppliers. Conceptually, these polymers are nothing else than poly-dentate ligands.
An obvious option for a type I polymeric ligand is poly(4-vinylpyridine) (P4VP) as substituted and non-substituted pyridines are omnipresent ligands in coordination chemistry and synthetic protocols for a huge number of pyridine metal complexes are known and are often extremely simple. Indeed, P4VP has been used in several studies for the preparation of metal-containing polymers. It is soluble in polar solvents and cheap. Examples for metals which have been used are Ru, Co, Ni, Zn, Cu, Os, Fe, Sn, Al, Cr, Mn, Mo, Pd, Hg, W, Ti, and rare earth metals [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Interestingly, to our knowledge there is only one publication on a gold-containing P4VP up to now. Rawashdeh-Omary et al. synthesized P4VP–Au–C6F5. They characterized the material by 1H NMR and luminescence spectroscopy. The luminescence is assigned to an excited state based on aurophilic interactions randomly formed in the polymeric materials [26].
In this contribution, we will present (P4VP)–AuCl and (P4VP)–ZnCl2 polymers and as model compound (Etpy)AuCl (with Etpy = 4-ethylpyridine). In addition to chemical characterization, we also investigated their photophysical properties.
Results and discussion
Synthesis
The metal-containing polymers are prepared by reaction of an ethanolic solution of P4VP (Mw = 60,000 g mol−1) with a DCM solution of (Me2S)AuCl (1, Scheme 1) and ZnCl2 (2), respectively. For 1, a molar ratio for pyridine:gold of 1:1 and for 2 an excess of 2:1 for pyridine:Zn were used. In both cases, a colorless precipitate was formed which was filtered off and dried. Under the assumption that besides AuCl and ZnCl2 no further constituents are present, the results of the C, H, N analysis indicate empirical formulas of (C7H7N)(AuCl)0.4 and (C7H7N)(ZnCl2)0.7.

The zinc polymer is not soluble in common organic solvents indicative of a high degree of cross-linking by the zinc cation. Zinc cations usually exist in tetrahedral coordination geometry. For zinc halide complexes, the standard composition is L2ZnX2 with two N-heterocyclic ligands L attached to the zinc cation. Hence, the Zn(II) atom can be coordinated by pyridine groups in an inter- or intrachain manner cross-linking the P4VP polymer. On the contrary, the (P4VP)–AuCl complex is soluble in DMSO allowing for recording a 1H NMR spectrum. In Fig. 1, the proton NMR of free P4VP is depicted showing broad resonances of the pyridine protons at 6.60 and 8.28 ppm. Upon coordination to the Au(I) atom, long tails at the high-field side of the signals of the pyridine moiety appear. These results are in accordance with the homologue (P4VP)Au(C6F5) polymer and the model compound 3 (vide infra) [26].
A para-substituted ethylpyridine can be regarded as a small molecule analog for P4VP. The model compound (Etpy)AuCl (3) will allow photophysical investigation of the pyridine–gold moiety also present in P4VP. Particularly, it can help to answer the question if luminescence is based on the isolated moiety or on an aggregation by π-π or aurophilic interactions. 3 was prepared from (Me2S)AuCl with 4-ethylpyridine in DCM. Interestingly, the slightly less reactive (tht)AuCl (tht = tetrahydrothiophene) does not give 3 as only the gold precursors can be isolated upon reaction with Etpy. The 1H NMR spectrum of 3 is as expected for an AA’BB’ spin system substituted with an ethyl group and corresponds also to the resonances of polymer 1.
Structural studies
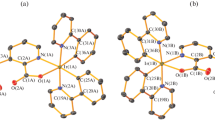
Crystals of 3 suitable for single-crystal X-ray diffraction were obtained by gas phase diffusion of diethyl ether into a solution of 3 in DCM at 4 °C. 3 crystallizes in the monoclinic space group P21/c with Z = 4. Interestingly, neither aurophilic contacts nor π-π stacking interactions are present. The shortest Au–Au distance is 3.962 Å and well beyond the accepted limit for aurophilic interactions. The gold atom is linearly coordinated by the nitrogen and chlorine atoms with an N–Au–Cl angle of 177.7(2) (Fig. 2). The Au–Cl and Au–N distances are 2.248(3) and 2.025(8) Å, respectively. These values are in accordance with reported values for py–Au–Cl [27, 28]. It should be noted that two forms of gold chloride complexes bearing various pyridine ligands are known. In their crystals, they are either found as neutral molecule py–Au–Cl as it is the case for 3 or in the ionic form [(py)2Au][AuCl2] [27,28,29,30,31,32]. The formation is dependent on the crystallization conditions and the pyridine ligand and has some relevance also for the structure of 1. However, we tried to recrystallize 3 in different solvents but we only could isolate the neutral form. Hence, it is tempting to postulate that 1 is also neutral.
Only a few red crystals of 4 were obtained from one attempt to grow crystals of 3. Obviously, it is formed by a disproportionation reaction as also some violet precipitate was formed. The latter is indicative of elemental gold. As it was not the goal of this study to synthesis pyridine–gold(III) complexes, no attempts to prepare pure 4 were undertaken. For a proper chemical analysis, the amount of formed materials was too low. However, we include the molecular structure for completeness and due to the fact that L–AuCl3 complexes bearing pyridine-like ligands L are relatively rare [33,34,35,36,37,38]. 4 crystallizes in the orthorhombic space group Pcab with eight formula units in the unit cell. The gold atom is surrounded by the nitrogen atom of the pyridine ligand and three chlorido ligands in a quadratic–planar coordination environment with an angular sum of exact 360.0° (Fig. 3). With 2.030(6) Å, the Au–N distance is slightly longer than in 3, as it is the case for the Au–Cl distances which are between 2.260(2) and 2.277(2) Å (Table 1). These values are comparable to those reported [33,34,35,36,37,38]. In addition, there are long Au…Cl contacts of 3.275(2) and 3.408(2) Å between the Au(III) atom and two chlorine atoms of two neighboring complexes forming an infinite chain (Fig. 3). These values are slightly below the sum of the van der Waals radii of Au (1.66 Å) and Cl (1.75 Å) of 3.41 Å. Considering these interactions, the gold atom exists in a sixfold coordination environment which is not unprecedented but relatively rare for Au(III) atoms [37, 39,40,41,42,43,44]. Between the pyridine moieties short π–π stacking interactions of ~ 3.6 Å are found.
Photophysical studies
In the solid state, both metallo-polymers clearly feature luminescence upon excitation with UV light. P4VP, however, is only very weakly emissive at 77 K (λmax ~ 430 nm). At room temperature, no authentic emission could be detected. This behavior is not surprising as it is similar to pyridine which is considered to be almost non-emissive in solution, because low-lying n–π* states efficiently quench emissions. At 77 K, pure crystalline pyridine as well as solutions of pyridine feature weak dual emission with a broad, structureless phosphorescence band (360–540 nm, λmax ~ 430 nm) [45]. In the case that the n–π* transition is blocked by protonation, a broad phosphorescence band could be detected around 400 nm even at room temperature in solution [46]. The emission of both the free pyridine as well as of the pyridinium cation was assigned to a 3π–π* excited state. Just recently, we have reported on nicotine–zinc halide complexes which feature phosphorescence in solid state at 77 K comparable to the pyridinium cation [47]. Nicotine is a naturally occurring pyridine derivative.
The P4VP–gold complex is soluble in DMSO but not in other common organic solvents. This fact prevents a detailed investigation of the photophysical properties in solution because DMSO absorbs already at ~ 300 nm. In solid state, the P4VP–gold complex shows intense emissions at ~ 490 nm both at room temperature as well as at 77 K (Fig. 4). In principle, the emission can either be based on excited states based on aurophilic interactions or on an intraligand (IL) excited state of the pyridine moiety as described above. For this reason, we investigated the luminescence behavior of the mono-nuclear complex 3 as a model. It is particularly helpful that according to the crystal structure, no close aurophilic or π–π interactions are present between the complexes. Therefore, emissions based on intermolecular interactions are not likely in solid state. Indeed, we could not detect authentic emissions either in solution or in solid at r.t. or 77 K. This observation is also in accordance with the absence of luminescence of py–Au–Cl reported previously [29]. As there are no clear and intense emissions associated with the molecular entity ‘py–Au–Cl’ in 3, we tentatively assign the emission band of the polymer 1 to excited states related to aurophilic interactions comparable to P4VP–Au–C6F5 reported by Rawashdeh-Omary et al. [26] and other pyridine–gold(I) complexes featuring close Au–Au contacts in the solid state [29, 31]. These interactions can be either intra- or interchain. However, for (P4VP)Au(C6F5) both the intensity as well as the emission wavelength are strongly dependent on the temperature and excitation wavelength. We could not detect a comparable behavior for 1. Due to insolubility of 1 in standard ‘spectroscopic’ solvents, no absorbance spectra could be recorded. The excitation spectra of neat 1 feature low-energy bands at λmax = 350 nm (r.t.) and below 350 nm (77 K). The free P4VP has an absorbance at 254 nm, complex 3 one of 243 nm each in DCM solution. Therefore, the bands in the excitation spectra could not be assigned to π–π* transition of the pyridine moiety. In fact, these values are in the standard range of other complexes with aurophilic contacts in the solid state [31, 48, 49]. Complex 4 absorbs at 243 and 316 nm. The low-energy band is typical for a Cl→Au ligand-to-metal charge transfer (LMCT) [50].
As already mentioned, some pyridine derivatives show photoluminescence upon blocking its quenching n–π* states. As demonstrated above for 3, not every Lewis acidic centers lead to emissive pyridine moieties. However, compound 2 is emissive in solid state both at room temperature and at 77 K. The emission spectra are highly dependent on the excitation wavelength. However, no systematic dependency between excitation wavelength and emission spectra could be identified. In Fig. 5, only one example is depicted. All emission bands are relatively broad covering a large fraction of the visible spectral region and show more than one maximum. This observation is similar not only to (P4VP)Au(C6F5) but also resembles the emission behavior of imidazolium-based ionic liquids. Like pyridinium, the imidazolium cation is a six-electron π system. The unusual luminescence properties have been explained by the existence of aggregated species which give rise to energetically different excited (and emissive) states with inefficient excitation energy-transfer process between them [51,52,53,54]. In an analogous interpretation, the zinc cations in P4VP might impose aggregation of pyridine chromophores. Dependent on their local environment and structures, excited states of different energies are formed. This would explain not only the excitation wavelength-dependent emission of 2 but also of (P4VP)Au(C6F5). For 1, the emissions which are based on metallophilic are obviously more intense dominating the emission spectrum and/or the aggregation by aurophilic interactions prevents the formation of emissive pyridine aggregates.
Conclusion
Au(I) and Zn(II) complexes containing the poly-dentate ligand poly(4-vinylpyridine) (P4VP) can be prepared by reaction between (Me2S)AuCl and ZnCl2, respectively. In both cases, almost insoluble substances with empirical formulas (PVP)(AuCl)0.4 and (PVP)(ZnCl2)0.7 are formed. Both materials feature luminescence upon excitation with UV light. Emissions of (P4VP)–AuCl are based on aurophilic interactions whereas for the (P4VP)–ZnCl2 compound, an excitation wavelength-dependent emission band could be observed. The latter behavior can be explained by the formation of aggregated species with different excitation energies. The model compound, (Etpy)AuCl, is not engaged in aurophilic or π–π interactions in the solid state. It does not show photoluminescence.
Experimental
All solvents and other reagents were commercially obtained and used as received. (Me2S)AuCl were prepared according to a published procedure [55]. P4VP was obtained from Sigma–Aldrich with a molar mass of Mn ≈ 60,000 g mol−1. NMR spectra were recorded on an Avance DRX 300 (300 MHz) spectrometer, and 1H shifts are reported in ppm relative to SiMe4, with the residual signal of the deuterated solvent as internal reference. UV–Vis spectra were recorded on a Cary 300 Bio photometer. The emission spectra were recorded on a Horiba Jobin–Yvon Fluorolog-3 spectrofluorometer. All emission spectra were corrected for wavelength-dependent instrument and detector response.
Single-crystal structure analysis was carried out on a Bruker Smart X2S diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The structures were solved by direct methods (SHELXS-97) [56] and refined by full-matrix least-squares on F2 (SHELXL-97) [57]. The H atoms were calculated geometrically, and a riding model was applied in the refinement process (Table 2). CCDC 1523478 and 1523479 contain the supplementary crystallographic data for 3 and 4. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre at https://summary.ccdc.cam.ac.uk/structure-summary-form. Mass spectra were collected on a Finnigan LCQ DecaXPPlus ion trap mass spectrometer with ESI ion source emission.
Chlorido[poly(4-vinylpyridine)]gold(I), (P4VP)AuCl (1, (C7H7N) AuCl)0.4)
An ethanolic solution of 17 mg poly(4-vinylpyridine) (0.17 mmol of pyridine groups) was added to 50 mg (Me2S)AuCl (0.17 mmol) in 20 cm3 dichloromethane. A clear solution formed. After few minutes, a white precipitate was formed. The reaction mixture was stirred for additional 2 h at room temperature. The product was filtrated yielding a white powder. Yield: 38 mg (76%).
Dichlorido[poly(4-vinylpyridine)]zinc(II), (P4VP)ZnCl2 (2, (C7H7N) (ZnCl2)0.7)
An ethanolic solution of 850 mg P4VP (8 mmol of pyridine groups) was added to 20 cm3 of an ethanolic solution of 220 mg ZnCl2 (1.6 mmol). A clear solution formed. After few minutes, a white precipitate was formed. The reaction mixture was stirred for additional 2 h at room temperature. The product was filtrated yielding a white powder. Yield: 180 mg (46%).
Chlorido(4-ethylpyridine)gold(I), (4-Etpy)AuCl (3, C7H9AuClN)
4-Ethylpyridine (1.0 mmol, 0.11 g) was added to a dichloromethane solution (20 cm3) of 0.30 g (Me2S)AuCl (1.0 mmol) and was stirred for 2 h at room temperature in the dark. After removing the solvent under vacuum, the residue was washed with diethyl ether to give a white product. Recrystallization by slow diffusion of diethyl ether into DCM solution of 3 yielded crystals suitable for single-crystal X-ray diffraction. Yield: 200 mg (58.82%); ESI–MS (MeOH): m/z = 411.27 ([(Etpy)2Au]+); 1H NMR (DMSO-d6, 300 MHz): δ = 1.19 (3H, t), 2.71 (2H, q), 7.59 (2H, m), 8.67 (2H, m) ppm; UV–Vis (DCM): λmax = 254 nm.
Trichlorido(4-ethylpyridine)gold(III), (4-Etpy)AuCl3 (4, C7H9AuCl3N)
Some red crystals of 4 were formed in an attempt to recrystallize 3 from DCM/Et2O. Also some purple precipitate was formed from the disproportionation reaction. The amount of 4 was not sufficient for a complete analysis. Besides single-crystal X-ray diffraction, an absorbance spectrum in DCM was recorded: UV–Vis (DCM): λmax = 243, 316 nm.
References
Manners I (ed) (2004) Synthetic metal-containing polymers. Wiley, Weinheim
Sheats JE, Carraher CE Jr, Pittman CU Jr, Zeldin M, Currell B (eds) (1990) Inorganic and metal-containing polymeric materials. Plenum Press, New York
Whittell GR, Hager MD, Schubert US, Manners I (2011) Nat Mat 10:176
Winter A, Schubert US (2016) Chem Soc Rev 45:5311
Chan WK (2007) Coord Chem Rev 251:2104
Ho C-L, Wong W-Y (2011) Chem Rev 255:2469
Abd-El-Aziz AS, Strohm EA (2012) Polymer 53:4879
Yan Y, Zhang J, Ren L, Tang C (2016) Chem Soc Rev 45:5232
Theis S, Iturmendi A, Gorsche C, Orthofer M, Lunzer M, Baudis S, Ovsianikov A, Liska R, Monkowius U, Teasdale I (2017) Angew Chem 129:16071
Dam ATV, Pijanowska D, Olthuis W, Bergveld P (2003) Analyst 128:1062
Belfiore LA, McCurdie MP, Das PK (2001) Polymer 42:9995
McCurdie MP, Belfiore LA (1999) Polymer 40:2889
Wu KH, Wang YR, Hwu WH (2003) Polym Degrad Stabil 79:195
Santana AL, Noda LK, Pires ATN, Bertolino JR (2004) Polym Test 23:839
Agnew NH (1976) J Polym Sci 14:2819
Biedermann H-G, Seidl P (1976) Makromol Chem 177:631
Caruso U, DeMaria A, Panunzi B, Roviello A (2002) J Polym Sci A Polym Chem 40:2987
Nishide H, Deguchi J, Tsuchida E (1976) Chem Lett 5(2):169
Lacroix PG, Lin W, Wong GK (1995) Chem Mater 7:1293
Creaven BS, Long C, Russell G (1988) Inorg Chim Acta 146:25
Belfiore LA, McCurdie MP, Ueda E (1993) Macromolecules 26:6908
Nishide H, Hata S, Tsuchida E (1978) Makromol Chem 179:1445
Khan AK, Rashid R, Hussain M, Yunus U, Akhtar Z, Ali S, Zahra A, Mir S, Ansari MT, Mehmood Z, Sajjad A, Murtaza G (2016) Arab J Sci Eng 41:105
Biedermann HG, Obwander J, Wichmann K (1972) Z Naturforsch B 27:1332
Biedermann HG, Wichmann K (1973) Z Naturforsch B 28:182
Rawashdeh-Omary MA, López-de-Luzuriaga JM, Rashdan MD, Elbjeirami O, Monge M, Rodríguez-Castillo M, Laguna A (2009) J Am Chem Soc 131:3824
Yip JHK, Feng R, Vittal JJ (1999) Inorg Chem 38:3586
Jones PG, Ahrens B (1998) Z Naturforsch B 53:653
Adams H-N, Hiller W, Strähle J (1982) Z Anorg Allg Chem 485:81
Freytag M, Jones PG (2000) Chem Commun 4:277
Lin JCY, Tang SS, Vasam CS, You WC, Ho TW, Huang CH, Sun BJ, Huang CY, Lee CS, Hwang WS, Chang AHH, Lin IJB (2008) Inorg Chem 47:2543
Monkowius U, Zabel M, Yersin H (2008) Inorg Chem Commun 11:409
Bourosh P, Bologa O, Simonov Y, Gerbeleu N, Lipkowski J, Gdaniec M (2007) Inorg Chim Acta 360:3250
Adams H-N, Strähle J (1982) Z Anorg Allg Chem 485:65
Mohammad-Nataj R, Abedi A, Amani V (2013) Synth React Inorg Metal-Org Nano-Met Chem 43:1375
Alizadeh R, Hafezeffati S, Amani V, Khavasi HR, Harms K (2015) Monatsh Chem 146:581
Kitteringham E, Zhou Z, Twamley B, Griffth DM (2018) Inorg Chem 57:12282
Martín-Santos C, Michelucci E, Marzo T, Messori L, Szumlas P, Bednarski PJ, Mas-Ballesté R, Navarro-Ranninger C, Cabrera S, Alemán J (2015) J Inorg Biochem 153:339
Willner H, Rettig SJ, Trotter J, Aubke F (1991) Can J Chem 69:391
Asprey LB, Jack KH, Kruse H, Maitland A (1964) Inorg Chem 3:602
Einstein FWB, Rao PR, Trotter J, Bartlett N (1967) Chem Soc 1967:478
Criado JJ, Lopez-Arias JA, Macias B, Fernandez-Lago LR, Salas JM (1992) Inorg Chim Acta 193:229
Muller MC (1974) Gold Bull 7:3940
Kriechbaum M, List M, Berger RJF, Patzschke M, Monkowius U (2012) Chem Eur J 18:5506
Ghoshal SK, Maiti AK, Kastha GS (1984) J Luminesc 31–32:541
Motten AG, Kwiram AL (1977) Chem Phys Lett 45:217
Hobbollahi E, Veselkova B, List M, Redhammer G, Monkowius U (2016) Z Naturforsch B 71:1269
Leitner S, List M, Monkowius U (2011) Z Naturforsch B 66:1255
Monkowius U, Zabel M, Fleck M, Yersin H (2009) Z Naturforsch B 64:1513
Hirtenlehner C, Krims C, Hölbling J, List M, Zabel M, Fleck M, Berger RJF, Schoefberger W, Monkowius U (2011) Dalton Trans 40:9899
Samanta A (2006) J Phys Chem B 110:13704
Paul A, Mandal PK, Samanta A (2005) J Phys Chem B 109:9148
Binetti E, Panniello A, Triggiani L, Tommasi R, Agostiano A, Curri ML, Striccoli M (2012) J Phys Chem B 116:3512
Paul A, Samanta A (2006) J Chem Sci 118:335
Brandys M-C, Jennings MC, Puddephatt RJ (2000) J Chem Soc Dalton Trans 24:4601
Sheldrick GM (1990) Acta Crystallogr A 46:467
Sheldrick GM (2008) Acta Crystallogr A 64:112
Acknowledgements
Open access funding was provided by Johannes Kepler University Linz. We thank the JKU and Prof. Knör (JKU) of their generous support for the experimental work. The NMR spectrometers were acquired in collaboration with the University of South Bohemia (CZ) with financial support from the European Union through the EFRE INTERREG IV ETC-AT-CZ program (project M00146, “RERI-uasb”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Heinz Falk on the occasion of his 80th birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hobbollahi, E., List, M. & Monkowius, U. Poly(4-vinylpyridine) as ligand for Au(I) and Zn(II) cations: luminescent metal-containing polymers. Monatsh Chem 150, 877–883 (2019). https://doi.org/10.1007/s00706-019-2382-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-2382-4