Abstract
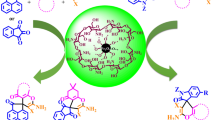
In the present study, we demonstrated the synthesis of magnetic cyanoguanidine-modified chitosan (MCGC) as an efficient and green retrievable heterogeneous nanocatalyst for one-pot three-component synthesis of benzimidazoloquinazolines (from 2-aminobenzimidazole, aromatic aldehydes, and dimedone) and 1,4-dihydropyridines (via Hantzsch-type condensation of ethyl acetoacetate, aromatic aldehydes, and ammonium acetate) under the ultrasonic irradiation and reflux conditions. The structure of the catalyst was fully confirmed using Fourier transform infrared spectroscopy, vibrating sample magnetometer, field emission scanning electron microscopy, energy dispersive spectroscopy, and thermogravimetric analysis. Increased amount of amino groups that are generated by modifying the surface of chitosan with cyanoguanidine as well as presence of hydroxyl groups determined the catalytic activity of MCGC. Furthermore, as experimental results confirmed, the ultrasonic-promoted reactions gave the better results in terms of reaction time, yield, and purity of isolated products. Cost effectiveness, mild conditions, low catalyst loading, convenient work-up, and ecofriendly solvent are some of the remarkable advantages of this protocol.
Graphic abstract








Similar content being viewed by others
References
Guin D, Baruwati B, Manorama SV (2007) Org Lett 9:1419
Sadeghzadeh SM (2016) Catal Lett 146:2555
Samoilova N, Krayukhina M, Naumkin A, Yamskov I (2018) Monatsh Chem 149:1179
Kasprzak A, Bystrzejewski M, Poplawska M (2018) Dalton Trans 47:6314
Anastas P, Eghbali N (2010) Chem Soc Rev 39:301
Pourjavadi A, Motamedi A, Hosseini SH, Nazari M (2016) RSC Adv 6:19128
Li D-D, Zhang J-W, Cai C (2018) Catal Commun 103:47
Dekamin MG, Azimoshan M, Ramezani L (2013) Green Chem 15:811
Shaabani A, Hezarkhani Z, Badali E (2015) RSC Adv 5:61759
Rao SN, Mohan DC, Adimurthy S (2014) Green Chem 16:4122
Shen C, Qiao J, Zhao L, Zheng K, Jin J, Zhang P (2017) Catal Commun 92:114
Lal J, Gupta SK, Agarwal DD (2012) Catal Commun 27:38
Zeng M, Zhang X, Shao L, Qi C, Zhang XM (2012) J Organomet Chem 704:29
Sudheesh N, Sharma SK, Shukla RS (2010) J Mol Catal A Chem 321:77
Kühbeck D, Saidulu G, Reddy KR, Díaz DD (2012) Green Chem 14:378
Abdelhamid IA (2009) Synlett 2009:625
Makhubela BC, Jardine A, Smith GS (2011) Appl Catal A 393:231
Bao Y, Shao L, Xing G, Qi C (2019) Int J Biol Macromol 130:203
Saikia G, Ahmed K, Gogoi SR, Sharma M, Talukdar H, Islam NS (2019) Polyhedron 159:192
Rangraz Y, Nemati F, Elhampour A (2018) Int J Biol Macromol 117:820
Chtchigrovsky M, Primo A, Gonzalez P, Molvinger K, Robitzer M, Quignard F, Taran F (2009) Angew Chem Int Ed 48:5916
Lee M, Chen B-Y, Den W (2015) Appl Sci 5:1272
Tap H, Sugimoto R (2018) Appl Surf Sci 434:188
Kumar S, Deepak V, Kumari M, Dutta P (2016) Int J Biol Macromol 84:349
Zhai X, Sun P, Luo Y, Ma C, Xu J, Liu W (2011) J Appl Polym Sci 121:3569
Maleki A, Firouzi-Haji R, Hajizadeh Z (2018) Int J Biol Macromol 116:320
Ramezanpour S, Panahi A, Rominger F (2018) Monatsh Chem 149:625
Palermo V, Sathicq ÁG, Constantieux T, Rodríguez J, Vázquez PG, Romanelli GP (2016) Catal Lett 146:1634
Neochoritis CG, Zhao T, Dömling A (2019) Chem Rev 119:1970
Dömling A (2006) Chem Rev 106:17
Kalinski C, Lemoine H, Schmidt J, Burdack C, Kolb J, Umkehrer M, Ross G (2008) Synthesis 2008:4007
Mamaghani M, Tabatabaeian K, Shirini F, Rassa M (2012) Bioorg Med Chem Lett 22:5956
Dandia A, Singh R, Bhaskaran S, Samant SD (2011) Green Chem 13:1852
Wu C, Lu L-H, Peng A-Z, Jia G-K, Peng C, Cao Z, Tang Z, He W-M, Xu X (2018) Green Chem 20:3683
Mason TJ (1997) Chem Soc Rev 26:443
O’Hagan D (2000) Nat Prod Rep 17:435
Alagarsamy V, Pathak US (2007) Bioorg Med Chem 15:3457
Alagarsamy V (2004) Pharmazie 59:753
Alagarsamy V, Murugananthan G, Venkateshperumal R (2003) Biol Pharm Bull 26:1711
Hour M-J, Huang L-J, Kuo S-C, Xia Y, Bastow K, Nakanishi Y, Hamel E, Lee K-H (2000) J Med Chem 43:4479
Alagarsamy V, Revathi R, Meena S, Ramaseshu K, Rajasekaran S, De Clerco E (2004) Indian J Pharm Sci 66:459
Smutny T, Nova A, Drechslerová M, Carazo A, Hyrsova L, Kunes J, Pour M, Špulák M, Pavek P (2016) J Med Chem 59:4601
Ahmad I (2017) MedChemComm 8:871
Tebbe MJ, Spitzer WA, Victor F, Miller SC, Lee CC, Sattelberg TR, McKinney E, Tang JC (1997) J Med Chem 40:3937
Charifson PS, Grillot AL, Grossman TH, Parsons JD, Badia M, Bellon S, Deininger DD, Drumm JE, Gross CH, LeTiran A (2008) J Med Chem 51:5243
White AW, Almassy R, Calvert AH, Curtin NJ, Griffin RJ, Hostomsky Z, Maegley K, Newell DR, Srinivasan S, Golding BT (2000) J Med Chem 43:4084
Naesdal J, Bodemar G, Walan A (1984) Scand J Gastroenterol 19:916
Kamali F, Shirini F (2017) New J Chem 41:11778
Sheldon R, Arends A, Hanefeld U (2007) Green chemistry and catalysis. Wiley, VCH Verlag GmbH and Co KgaA, Weinheim
Heravi MM, Derikvand F, Ranjbar L (2010) Synth Comm 40:677
Gajaganti S, Kumari S, Kumar D, Allam BK, Srivastava V, Singh S (2018) J Heterocycl Chem 55:2578
Boecker RH, Guengerich FP (1986) J Med Chem 29:1596
Sirisha K, Bikshapathi D, Achaiah G, Reddy VM (2011) Eur J Med Chem 46:1564
Klusa V (1995) Drugs Future 20:135
Bretzel RG, Bollen CC, Maeser E, Federlin KF (1993) Am J Kidney Dis 21:S53
Janis RA, Triggle D (1983) J Med Chem 26:775
Mager P, Coburn R, Solo A, Triggle D, Rothe H (1992) Drug Des Discov 8:273
Itoh T, Nagata K, Miyazaki M, Ishikawa H, Kurihara A, Ohsawa A (2004) Tetrahedron 60:6649
Dondoni A, Massi A, Minghini E, Bertolasi V (2004) Tetrahedron 60:2311
Cherkupally SR, Mekala R (2008) Chem Pharm Bull 56:1002
Safari J, Banitaba SH, Khalili SD (2011) J Mol Catal Chem 335:46
Albadi J, Shirini F, Ghabezi B, Seiadatnasab T (2017) Arab J Chem 10:S509
Dekamin MG, Kazemi E, Karimi Z, Mohammadalipoor M, Naimi-Jamal MR (2016) Int J Biol Macromol 93:767
Mahinpour R, Moradi L, Zahraei Z, Pahlevanzadeh N (2018) J Saudi Chem Soc 22:876
Kumar P, Kadyan K, Duhan M, Sindhu J, Hussain K, Lal S (2019) Chem Pap 73:1153
Davarpanah J, Ghahremani M, Najafi O (2019) J Mol Struct 1177:525
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:1240
Khaskel A, Barman P (2016) Heteroat Chem 27:114
Aftan MM (2018) Tikrit J Pure Sci 23:83
Hemmati B, Javanshir S, Dolatkhah Z (2016) RSC Adv 6:50431
Mousavi MR, Maghsoodlou MT (2015) J Iran Chem Soc 12:743
Yao C, Lei S, Wang C, Li T, Yu C, Wang X, Tu S (2010) J Heterocycl Chem 47:26
Weber L (2002) Drug Discov Today 7:143
Mousavi MR, Maghsoodlou MT (2014) Monatsh Chem 145:1967
Suslick KS, Hammerton DA, Cline RE (1986) J Am Chem Soc 108:5641
Tameh FA, Safaei-Ghomi J, Mahmoudi-Hashemi M, Shahbazi-Alavi H (2016) RSC Adv 6:74802
Maleki A, Rahimi J (2018) J Porous Mat 25:1789
Amoozadeh A, Rahmani S (2015) J Mol Catal A Chem 396:96
Acknowledgements
We are thankful for the financial support from Chemical Injuries Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javanmiri, K., Karimian, R. Green synthesis of benzimidazoloquinazolines and 1,4-dihydropyridines using magnetic cyanoguanidine-modified chitosan as an efficient heterogeneous nanocatalyst under various conditions. Monatsh Chem 151, 199–212 (2020). https://doi.org/10.1007/s00706-019-02542-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02542-z