Abstract
Metabolism of numerous elements is involved in carcinogenesis. Among other, calcium and zinc metabolic pathways coincide with prostate cancer development. It is probable that the restoration of elemental loses can moderate or even stop prostate malignancy. For this reason, studies on elemental content in tissues taken from organisms with cancer are carried out to establish proper supplementation. ICP-MS is a well-established method for the analysis of such a kind of samples. The study aimed to investigate the influence of dietary supplementation on elemental content in the prostate gland of rats with implanted cancer cells. Tested animals were divided into six dietary groups: standard diet and supplementation with Ca, Fe, Zn, Cu, or Se. Every dietary group was divided into experimental group (with implanted cancer cells) and control group (without cancer cells). Prostate tissue was dried and treated with microwave-assisted mineral digestion. Total elemental content was quantified by ICP-MS. Student's t test and analysis of variance with post-hoc Tukey’s procedure were applied to compare treatment and dietary groups. The total content of selected elements (K, Na, Mg, Ca, Fe, Zn, Cu, Sr, Pb, and Se) was quantified, with statistically significant differences in Na, Mg, Ca, Zn, Sr, and Se contents between treatment groups and in Ca, Fe, Cu, Zn, Sr, and Pb contents between diet groups. High heterogeneity within group could be observed for Zn content in the tissue, confirming the crucial role of zinc in prostate gland metabolism and pointing its inter-individual variability.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer (malignant neoplasm) remains the second cause of death in Europe [1]. According to European Cancer Information System, prostate cancer is the most common type of cancer in European men [1]. Three risk factors of prostate cancer are well-established: age, race/ethnicity, and family history related to inherited genetic factors [2, 3]. However, owing to its slow progression with low latency period, prostate cancer is a good candidate for investigating lifestyle factors, inhibiting malignancy development. In the early stage, patients are often under the so-called active surveillance (AS)—medical care basing on watching a patient’s condition but not giving any treatment unless the patient is getting worse [4]. A lot of dietary factors is presumed to be linked with this type of cancer, but there is still no clear dietary recommendation for patients under AS [4].
Genetic risk factors related to metabolism
Black race favors both prostate cancer morbidity and mortality. The highest prostate cancer incidence worldwide is observed in African–American men and in Caribbean countries inhabitants of African descent, mainly in Jamaica and Trinidad and Tobago [5–7]. In the United States, in the years 2008–2012, the average annual prostate cancer incidence rate was 70% higher in black men than in white men [6]. Similar disparities between races are observed for mortality. In that case, it should be taken into account that death rates for the majority of cancers are higher among the black race than among the white race; however, for prostate cancer, disproportion is almost the highest (after stomach cancer) [6]. There are a lot of studies looking for a cause of this phenomenon and the skin color theory seems to be the most probable. The darker skin is, it contains more melanin that inhibits calcitriol synthesis and decreased level of this vitamin D metabolite is well-known risk factor of prostate cancer [8]. Not only genetics plays a role in prostate cancer development, because in migration studies, it was noted that incidence rate in Asian men increases significantly after they move to the United States [9].
Lifestyle risk factors in prostate cancer
Increase of the prostate cancer incidence risk after moving to developed country is directly linked to the lifestyle factors, such as physical activity and diet. In the prostate cancer lifestyle trial, it was stated that proper lifestyle (low-fat, plant-based diet, physical activity, and stress management) allows avoiding or delaying conventional treatment for at least 2 years [10]. First general nutritional factor is high-energy intake, often connected with poor nutrition value, leading to obesity and metabolic syndrome. Obesity favors many malignancy processes, prostate cancer included. In metabolic syndrome, the most significant for prostate cancer development are insulin-related features such as hyperinsulinemia, insulin resistance, and raised IGF-1 (insulin-like growth factor 1) [11]. Fat tissue deposition is also taken into consideration, and the crucial role of peri-prostatic adipose tissue is underlined in the literature, as its quantity is associated with increased prostate cancer aggressiveness [12]. In the systematic review of prospective cohort studies from 2014, it was concluded that obesity increased the risk of fatal prostate cancer, but its influence on prostate cancer incidence remained unclear [13]. A possible explanation is the fact that greater plasma volume in obesity causes the dilution of PSA concentration and, therefore, delays diagnosis [9]. Peri-prostatic adipose tissue area or density were even more clearly correlated with prostate cancer aggressiveness than typical obesity indicators such as BMI (body mass index) or waist circumference [12]. Obesity and metabolic syndrome are linked with fat consumption, but similar to cardiovascular diseases, its influence on prostate cancer is dependent on type of fat. Saturated fatty acids and trans-isomers are associated with increased prostate cancer risk, while polyunsaturated n-3 fatty acids can reduce the risk of the disease and extend the survival [11]. The mechanism of the protective effect of n-3 fatty acids is ascribed to the anti-inflammatory effects (as a result of the advisable n-3 to n-6 ratio), enhancing whole immune system functions, and to the specific activity directed to cancer cells: antiproliferative and inducing apoptosis [14]. Widely discussed is the influence of calcium and vitamin D metabolism on prostate cancer development. Calcium intake should be specified by its origin from dairy or non-dairy products. Excessive dairy products consumption is presumed to be associated with prostate cancer developing, by multiple mechanisms, such as elevated fat intake [15], suppression of circulating calcitriol which inhibits cancer cells proliferation and induces apoptosis [16], or increasing in IGF-1 from milk containing oestrogen [15]. In the meta-analysis of 32 prospective studies, it was stated that the intake of dairy products and dietary calcium was associated with increased total prostate cancer risk [17]. Nondairy calcium and supplemental calcium intake were not associated with total cancer risk, but supplemental calcium correlated positively with fatal prostate cancer morbidity [17]. According to systematic review from 2013, milk intake, particularly during adolescence, appears to positively correlate with the risk of prostate cancer; however, authors found no definite data about the effect of milk intake on tumor progression [15]. Highly related with calcium metabolism is vitamin D, and the correlation between calcitriol deficiency and prostate cancer risk is observed, while higher serum levels of calcitriol are associated with better prognosis and improved outcomes [18]. Other highlighted element in prostate cancer studies is selenium. In the large multicenter clinical trial Selenium and Vitamin E Cancer Prevention Trial (SELECT) [19], no statistically significant differences were observed between four groups receiving: vitamin E, selenium, both compounds, and placebo. Moreover, high doses of selenium are considered as carcinogenic [20]. Other dietary compounds are also discussed, as well as specific types of products (tomatoes, cruciferous vegetables, pomegranate, and green tea), but the evidence is not enough to formulate concrete recommendations [15]. On the cellular level, it is proven that prostate cells are able to accumulate high content of zinc, but during the malignant process, they lose this ability and it is probable that the restoration of high zinc levels can moderate or even stop prostate malignancy [21]. For this reason, studies on elemental content in tissues taken from organism with cancer are carried out to establish proper supplementation or dietary recommendations.
Chemical analysis of prostate gland elemental content
Kiziler et al. [22] discussed the possibility of using levels and ratio changes of trace elements in tissue for prostate cancer diagnostic process. Among other elements, the role of cadmium, selenium, zinc, iron, and copper has been underlined [22]. Zaichick et al. [23] quantified 43 trace elements in normal, benign hyperplastic, and carcinomatous human prostate gland taken during biopsy or prostatectomy from 92 patients, using neutron activation analysis and inductively coupled plasma mass spectrometry (ICP-MS). Authors noticed significant differences in Cd to selected elements content ratios between groups and proposed introducing this method as an accurate tool for diagnosing prostate cancer. However, quantification and calculation of this ratio were not always possible because of the insufficient weight of the needle biopsy material to perform the analysis [23]. In the earlier study, authors established the method and revealed statistical differences between elemental content of prostate gland in different age groups [24]. Nyman et al. [25] have proposed total selenium and selenomethionine analysis using high-performance liquid chromatography and ICP-MS. Only carcinomatous tissue has been analyzed, with peripheral and transitional zones comparison; in peripheral zone, significantly higher total selenium level was quantified. Leitão et al. [26] used total reflection X-ray fluorescence to determine P, S, K, Ca, Fe, Cu, Zn, and Rb finding significant differences between normal and carcinomatous human prostate gland in S, Fe, and Zn concentrations. Determination of trace elements in complex matrices, such as clinical samples, is still a challenging task due to their extremely low concentrations and significant matrix effects. For a long time, graphite furnace atomic absorption spectrometry (GF AAS) and instrumental neutron activation analysis (INAA) have been commonly used for the purpose. However, INAA is nowadays not recommended due to the problematic sample storage after the analysis including irradiation and activation. GF AAS becomes time-consuming if the analysis of many elements is needed. Taking into account the literature data [27, 28], an ICP-MS method and inductively coupled plasma optical emission spectrometry (ICP-OES) can be distinguished among other analytical techniques as a powerful tool in multielemental analysis in a wide range of concentrations, with high sensitivity, short time of analysis, and low limits of quantitation. Applications of ICP-MS and ICP-OES have been reported for the wide range of biological and clinical samples, both fluids (blood [29], amniotic fluid [30], and cerebrospinal fluid [31]) and solid tissues [31–36]. Complex matrices of clinical samples are often a source of interferences caused by monoatomic and polyatomic ions, formed in the plasma from the matrix constituents. Among other solutions, mathematical corrections are commonly used to minimize interferents influence and matrix-matched certified reference materials (CRM) are used to verify the trueness of the results. ICP-MS combined with efficient digestion procedure offers reliable results for small samples, such as rat prostate gland. Preliminary trace-metal analysis is also possible to check specific features of the sample. In this study, ICP-MS was chosen as an analytical method to investigate the influence of dietary supplementation on elemental content in the prostate gland of rats with implanted cancer cells.
Results and discussion
Experimental/control group comparison
Mean values with the standard deviations for the content of ten elements in rat prostate glands are presented in Table 1, separately for different diet groups and for different treatment groups: experimental (with implanted cancer cells) and control (without implanted cells). Results were compared with similar studies on human prostate. In general, obtained elemental content values were in the same order of magnitude as in Zaichick et al. studies [23, 37], as well as in Leitão et al. study [26], except for Zn content, which was significantly higher in both authors' studies than in the presented research. These discrepancies can indicate significant differences between the Zn content in the rat and human prostate gland. Obtained selenium contents, compared with Nyman et al. study [25], were also in agreement.
Elemental content ratios between experimental and control groups are presented in Table 2. Significant changes between experimental and control group were observed for Na, Ca, Zn, and Sr in standard diet group and for Mg and Se in Ca-supplemented diet group.
Fluctuations in electrolytes content could be expected, because Na+ and Ca2+ channels are expressed or even overexpressed in prostate cancer [38]. As a consequence, signal transduction in cells could influence temporary changes in Na+ and Ca2+ content. Sr is a well-known calcium biological analogue [39]. Thus, Sr content and proportions in tissue can be the reflection of Ca content. As for Mg2+, it is the second intracellular cation (after potassium), gating Ca2+ channels and acting as a physiological calcium antagonist [40]. Ca supplementation could influence Mg content more significantly in experimental than in control group.
Alkali ions’ content is relatively instable and sensitive to sample preparation, because the majority of their salts are very well soluble in water. These ions are used to be called "spectator ions", seeing that in many chemical reactions, they are only counterions, not involved in the essence of the process (for example, in precipitation). However, the role of alkali ions is crucial in biological systems. Different ionic radii and hydration sphere formations for K+ and Na+ allow selective transport of only one of these ions through the appropriate ion channel that enables the regulation of biological processes with high specificity [41]. Therefore, despite the undoubted relationship between K+ and Na+ concentration, the variation of only one of these elements in a specific part of the tissue is also possible, that was observed in the presented study, where only sodium concentration decreased significantly in experimental group compared to control (on Standard diet).
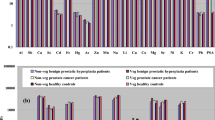
In Fig. 1, selected ratios from Table 2 between experimental and control group are presented, reflecting the influence of the supplemented element on its content in the prostate gland. Ratios above 1 mean that the tissue from experimental group is enriched in the investigated element comparing to control group.
The greatest (but not necessarily the most significant) differences were observed in zinc group supplementation. Decreased zinc level in the prostate gland was observed only for the standard diet group and for Zn-supplemented group; however, in the second case, the difference was not statistically significant. Lack of significance, despite the visible differences, was influenced by the high values of inside-groups standard deviations. Similarly high variance was observed in Kiziler et al.’s research [22]. This inhomogeneity can be explained by zinc accumulation by prostate gland cells and its metabolic involvement in the prostate carcinogenesis [21]. It should be considered that Zn content in the prostate gland can be highly inter-individual variable. Anyway, the accumulation of supplemented Zn was observed in the control group (the highest content among all of the groups), while in the experimental group, this phenomenon was stopped, probably by the malignant process. As a result, in Zn-supplemented group, Zn content ratio (experimental to control) was 0.3. It is in agreement with the fact that prostate cells are able to accumulate high content of zinc, and during the malignant process, they lose this ability [21].
Comparative studies of elemental content in normal and cancerous prostate tissue were carried out in humans. Zaichick et al. [37] compared prostate tissue taken from healthy men and from patients with adenocarcinoma and observed significantly lower levels of K, Na, Ca, and Mg in cancerous than in normal tissue. These results are partly in agreement with the presented study, where K, Na, Ca, and Mg levels were lower in experimental groups in case of the standard diet and for Na and Ca statistical significance of that difference was achieved. Mg content was significantly lower in experimental group compared to control in case of Ca-supplemented diet. However, other supplementation investigated in the study (Fe, Zn, Cu, and Se) seemed to reduce this tendency—experimental to control ratios in electrolytes content approached to 1. In the ensuing study, Zaichick et al. [23] determined trace elements in human normal and cancerous prostate tissue, comparing Cd/trace-element ratio. Authors observed significant differences among other in Fe, Pb, and Se contents, while in the presented study, Fe and Pb did not differ significantly between experimental and control groups, and in case of Se content, only in Ca-supplemented group, significant differences in Se levels were stated between experimental and control animals. In Zaichick et al.’s study [23], selenium content was increased in benign tumor and decreased in prostate cancer; Cd/Se ratio was decreased both in benign and malignant hyperplasia. In the presented study, in Ca-supplemented group, Se content was significantly higher in experimental than in control tissue. However, it should be taken into account that selenium content is on the very low level in prostate gland; thus, the results might be influenced by greater uncertainty. In Leitão et al.’s study [26], decreased levels in cancerous tissue compared to normal were stated for K, Ca, Fe, and Zn, that is compatible with the presented study in case of Ca and Zn for the standard diet group. Kiziler et al. [22] observed significantly lower Zn content in tissue with malign carcinoma compared to the benign hypertrophy (there was no control group for tissue analysis), similarly as in this study in the standard diet group. As it can be seen, literature data are in many cases in agreement with the achieved results. Human studies are more representative for clinical practice; however, it is difficult to perform reliable investigation concerning nutrition influence because of the differences in the usual diet of people. Even if patients receive identical food during research time, their history of nutrition is always different and cannot be under control. For this reason, supplementation influence was investigated on the animal model.
Diet groups’ comparison
Multi-factor ANOVA test with post-hoc Tukey's procedure was performed to compare different dietary groups. Statistically significant differences (p < 0.05) were presented only for the identical treatment groups (i.e., between two experimental groups or between two controls) in Fig. 2.
The content of the majority of main electrolytes (Na, K, and Mg) did not vary between dietary groups. However, significant differences were observed in Ca content for Cu- and Se-supplemented groups compared to the standard diet group. As for microelements, significantly higher levels of Fe, Cu, and Sr contents for Cu- and Se-supplemented groups compared to the standard diet group were stated. Pb content was significantly higher in Se-supplemented group and Zn content was significantly higher in Cu-supplemented group than in the standard diet group. The most of differences between groups were observed in Cu content, where Cu and Se supplementation induced significant changes not only in comparison with Standard diet group, but also with other dietary treatments. Visible accumulation of supplemented elements can be stated in case of Fe and Zn, where the supplementation is reflected in the increased levels of these elements, however, only in control groups.
Cu, Fe, and Sr contents were chosen to visualize the correlation in the 3D graph (Fig. 3). The tendency of grouping the animals with the same diet is observed, especially for standard diet (orange points) and Ca-supplemented diet (blue points). In case of Se-supplemented group (yellow points), the highest levels of highlighted elements are visible (comparing with other groups) and the individual correlation between Sr, Cu, and Fe contents is observed. It can be supposed that increased levels of one of these elements in the prostate gland tissue may be related to another one content.
In the study, elemental analysis of rat prostate gland was performed, including both main and trace elements on the relatively large group (n = 88), allowing the comparison with similar studies on human prostate tissue. As for authors' knowledge, for the first time, ICP-MS method was used for such a complex analysis, taking into account prostate cancer cells implantation as well as five different dietary interventions. Low limits of quantitation obtained in ICP-MS analysis have given the opportunity to compare trace-element content in low weight samples. The possibility of quantifying many isotopes during one measurement cycle and wide working range allowed collating electrolytes (Na, K, Mg, and Ca) content with Fe, Cu, Zn, Se, Sr, and Pb contents represented in significantly lower amounts.
Conclusion
Inductively coupled plasma mass spectrometry was used for multielemental analysis of rat prostate tissue. In the presented study, ten elements (K, Na, Ca, Mg, Fe, Zn, Cu, Se, Sr, and Pb) were quantified. Prostate gland elemental content varied dependently of the disease conditions (presence or absence of implanted prostate cancer cells) and of the diet, especially supplemented with Cu or Se. High heterogeneity within group could be observed for Zn content in the tissue, confirming the crucial role of zinc in prostate gland metabolism and pointing its inter-individual variability. Significant differences in calcium content between treatment groups remain compatible with its metabolic pathway linked with prostate gland development. For the first time, such a complex multielemental analysis of the rat prostate gland, including two kinds of intervention (cancer cells implantation as well as five different diet supplementations) has been performed, with achieving statistically significant differences between groups. The presented study may be a starting point for planning interventional clinical trials; however, more pre-clinical research should be done to avoid supplementation promoting carcinogenesis.
Experimental
Chemicals and reagents
Stock solutions were prepared by diluting ICP multi-element standard Merck VI (Merck, Germany). Certified Reference Material (CRM) MODAS-4 Cormorant Tissue was obtained from LGC Standards (Poland). For sample digestion, 69% nitric acid, analytical grade, was used (Merck, Germany). Samples, CRM, and standards were diluted with deionized water obtained by Milli-Q System (Merck, Millipore, Germany).
Instrumentation
Samples were dried in Drying Oven SLN 240 (Pol-Eko, Poland). The microwave Ethos Up closed system (Milestone, Italy) was used for mineral digestion of samples. Isotope-specific detection was achieved using quadrupole mass spectrometer with inductively coupled plasma ionization, ICP-MS (Nexion 300D, Perkin Elmer Sciex, USA), equipped in quartz cyclonic spray chamber, Meinhard nebulizer, and platinum skimmer cones. The working conditions of spectrometer were optimized daily to obtain the maximal sensitivity and stability as well as the lowest level of oxides and double charged ions. For all measurements, radio frequency plasma power was set to the value 1350 W and the constant nebulizer gas (argon) flow of 0.9 dm3 min−1 was used. Transient signals of the selected isotopes (23Na, 24 Mg, 39 K, 43Ca, 57Fe, 63Cu, 66Zn, 82Se, 88Sr, and 208Pb) were monitored (3 readings/1 sweep/5 replicates) with a dwell time 50 ms/isotope.
Material
Male Sprague–Dawley rats (n = 88) were obtained from the Animal Laboratory, Department of General and Experimental Pathology from the Medical University of Warsaw. The study was approved by the Ethics Committee, Medical University of Warsaw. Tested animals were housed in the standard conditions with 12-h light–dark cycle in 22 °C. Rats were fed with the standard diet (Labofeed H, Kcynia, Poland) and water ad libitum. The diet contained the following compounds (per 1 kg): protein (210 g), fat (39.2 g), fiber (43.2 g), ash (55 g), carbohydrates (300 g), vitamin A (15,000 IU), vitamin D3 (1000 IU), vitamin E (90 mg), vitamin K3 (3 mg), vitamin B1 (21 mg), vitamin B2 (16 mg), vitamin B6 (17 mg), vitamin B12 (80 μg), pantothenic acid (30 mg), folic acid (5 mg), nicotinic acid (133 mg), Ca (10 g), P (8.17 g), Mg (3 g), K (9.4 g), Na (2.2 g), Cl (2.5 g), S (1.9 g), Fe (250 mg), Mn (100 mg), Zn (76.9 mg), Cu (21.3 mg), Co (2.0 mg), I (1.0 mg), and Se (0.5 mg).
Experimental procedure
The experiment was carried out during 90 days. After 10 days of the adaptation period (from 60 to 70th day of rats' age), animals were randomly divided into experimental group (with implanted prostate cancer cells) and control group (without cancer cells). Control group was accommodated under the same conditions as experimental group and fed with the same diet. The cancer cells (LNCaP) were implanted intraperitoneally in the amount 1 × 106 in 0.4 cm3 of phosphate-buffered saline to the rats at day 90th of their lifetime. The certified line of androgen-dependent human prostate cancer cells was obtained from ATTC bank (American Type Culture Collection, Menassas, VA). Experimental and control animals were divided into dietary groups and supplemented with minerals by oral gavage (Table 3).
The rats were fed with 0.4 cm3 of supplemented suspension daily, from 70th until 150th day of their lifetime. The animals fed only with the standard diet (without supplementation) received 0.4 cm3 of water. The doses of trace elements were selected basing on the values used in the Labofeed H diet (extrapolated on the rats’ body weight). According to the level of trace elements in the Labofeed diet, the rats were fed, via gavage, extra supplements of the following: double dose of Zn and Cu or one dose of Fe or a quarter dose of Ca. The doses of selected minerals were chosen based on their levels in dietary supplements for humans.
Sample treatment
Prostate tissue collected from rats was dried in laboratory oven (24 h, 37 °C). Then, samples were weighted accurately (up to 0.0001 g) and treated with 69% nitric acid and deionized water (v/v 1:2). The mixture was digested in Teflon vessels, 15 min up to 180 °C and 10 min in 180 °C. After cooling down, the digests were diluted with deionized water. Total elemental content was quantified by means of ICP-MS. Quantitation was achieved by 5-point external calibration (standards from 1 μg dm−3 to 100 μg dm−3 for Cu, K, Mg, Na, Pb, and Sr; standards from 10 μg dm−3 to 1000 μg dm−3 for Fe, Se, and Zn; and standards from 100 μg dm−3 to 10,000 μg dm−3 for Ca). CRM was treated in the same way as prostate gland samples and recoveries were calculated, remaining as follows: Na 91%, Mg 101%, K 100%, Ca 99%, Fe 101%, Cu 97%, Zn 93%, Se 88%, Sr 92%, and Pb 110%. Limits of quantitation (LOQ) were calculated for each element based on the formula recommended by IUPAC [42] (Table 4).
Data treatment
Microsoft Excel 2010 software (Microsoft, USA) with XLSTAT add-on was used for statistical calculations. Outstanding results were eliminated by using Dixon’s test. For each group, mean value and standard deviation were calculated. Student's t test was applied to compare experimental groups with controls inside each dietary group. Values of p below 0.05 were considered to be statistically significant. Then, multi-factor analysis-of-variance (ANOVA) test was performed to compare dietary groups. In case of statistically significant difference (p value below 0.05), Tukey's post-hoc test was run to find which means are significantly different from each other. The comparison was performed for each element to check if different diets may influence the elemental composition of the prostate gland.
References
ECIS—European Cancer Information System © (2019) European Union. https://ecis.jrc.ec.europa.eu. Accessed 28 Feb 2019
Smith CJ, Minas TZ, Ambs S (2018) Am J Pathol 188:304
Panigrahi GK, Praharaj PP, Kittaka H, Mridha AR, Black OM, Singh R, Mercer R, van Bokhoven A, Torkko KC, Agarwal C, Agarwal R, Abd Elmageed ZY, Yadav H, Mishra SK, Deep G (2019) Cancer Med 8:1110
Gregg JR, Zheng J, Lopez DS, Reichard C, Browman G, Chapin B, Kim J, Davis J, Daniel CR (2019) Br J Cancer 120:466
Rebbeck TR, Devesa SS, Chang B-L, Bunker CH, Cheng I, Cooney K, Eeles R, Fernandez P, Giri VN, Gueye SM, Haiman CA, Henderson BE, Heyns CF, Hu JJ, Ingles SA, Isaacs W, Jalloh M, John EM, Kibel AS, Kidd LR, Layne P, Leach RJ, Neslund-Dudas C, Okobia MN, Ostrander EA, Park JY, Patrick AL, Phelan CM, Ragin C, Roberts RA, Rybicki BA, Stanford JL, Strom S, Thompson IM, Witte J, Xu J, Yeboah E, Hsing AW, Zeigler-Johnson CM (2013) Prostate Cancer 2013:560
DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A (2016) CA Cancer J Clin 66:290
Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F (2012) Eur Urol 61:1079
Giammanco M, Di Majo D, La Guardia M, Aiello S, Crescimannno M, Flandina C, Tumminello FM, Leto G (2015) Pharm Biol 53:1399
Schultz C, Meier M, Schmid HP (2011) Maturitas 70:339
Frattaroli J, Weidner G, Dnistrian AM, Kemp C, Daubenmier JJ, Marlin RO, Crutchfield L, Yglecias L, Carroll PR, Ornish D (2008) Urology 72:1319
Patel VH (2014) Expert Rev Anticancer Ther 14:1295
Nassar ZD, Aref AT, Miladinovic D, Mah CY, Raj GV, Hoy AJ, Butler LM (2018) BJU Int 121:9
Gołąbek T, Bukowczan J, Chłosta P, Powroźnik J, Dobruch J, Borówka A (2014) Urol Int 92:7
Gu Z, Suburu J, Chen H, Chen YQ (2013) Biomed Res Int 2013:824563
Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME (2014) Nutr Metab 11:30
Moreno J, Krishnan AV, Peehl DM, Feldman D (2006) Anticancer Res 26:2525
Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T (2015) Am J Clin Nutr 101:87
Swami S, Krishnan AV, Feldman D (2011) Mol Cell Endocrinol 347:61
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr, Baker LH, Coltman CA Jr (2009) JAMA J Am Med Assoc 301:39
Marshall JR, Tangen CM, Sakr WA, Wood DP Jr, Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF, Lee WR, Gaziano JM, Crawford ED, Ely B, Ray M, Davis W, Minasian LM, Thompson IM Jr (2011) Cancer Prev Res 4:1761
Costello LC, Franklin RB (2006) Mol Cancer 15:5
Kiziler AR, Aydemir B, Guzel S, Alici B, Ataus S, Tuna M, Durak H, Kilic M (2010) Trace Elem Electrolytes 27:65
Zaichick V, Zaichick S (2017) Cancer Ther Oncol 4:555626
Zaichick V, Zaichick S (2014) J Radioanal Nucl Chem 301:383
Nyman DW, Stratton MS, Kopplin MJ, Dalkin BL, Nagle RB, Gandolfi AJ (2004) Cancer Detect Prev 28:8
Leitão RG, Palumbo A, Souza PAVR, Pereira GR, Canellas CGL, Anjos MJ, Nasciutti LE, Lopes RT (2014) Radiat Phys Chem 95:62
Evans EH, Pisonero J, Smith CMM, Taylor RN (2018) J Anal At Spectrom 33:684
Su CK, Sun YC (2015) J Anal At Spectrom 30:1689
Czauderna M, Białek M, Krajewska K, Ruszczyńska A, Bulska E (2017) J Anim Feed Sci 26:192
Markiewicz B, Sajnóg A, Lorenc W, Hanć A, Komorowicz I, Suliburska J, Kocyłowski R, Barałkiewicz D (2017) Talanta 174:122
Ha Y, Tsay OG, Churchill DG (2011) Monatsh Chem 142:385
Lavilla I, Costas M, Miguel PS, Millos J, Bendicho C (2009) Biometals 22:863
Ek KH, Morrison GM, Lindberg P, Rauch S (2004) Arch Environ Contam Toxicol 47:259
Zhu H, Wang N, Zhang Y, Wu Q, Chen R, Gao J, Chang P, Liu Q, Fan T, Li J, Wang J, Wang J (2010) Health Phys 98:61
Czauderna M, Kowalczyk J, Bulska E, Boldižarova K, Niedźwiedzka KM, Ruszczyńska A, Leng L (2005) J Anim Feed Sci 14:529
Czauderna M, Kowalczyk J, Niedźwiedzka K, Wąsowska I, Pająk J, Bulska E, Ruszczyńska A (2004) J Anim Feed Sci 13:105
Zaichick V, Zaichick S (2016) J Hematol Oncol Res 2:20
Li M, Xiong Z-G (2011) Int J Physiol Pathophysiol Pharmacol 3:156
Kossman SE, Weiss MA (2000) Cancer 3:156
Fawcett WJ, Haxby EJ, Male DA (1999) Br J Anaesth 83:302
Kim Y, Nguyen TT, Churchill DG (2016) Met Ions Life Sci 16:1
Currie LA (1995) Pure Appl Chem 67:1699
Acknowledgements
The study was carried out at the Biological and Chemical Research Centre, University of Warsaw established within the project co-financed by European Union from the European Regional Development Fund under the Operational Programme Innovative Economy 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jagielska, A., Ruszczyńska, A., Wagner, B. et al. ICP-MS analysis of diet supplementation influence on the elemental content of rat prostate gland. Monatsh Chem 150, 1681–1690 (2019). https://doi.org/10.1007/s00706-019-02473-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02473-9