Abstract
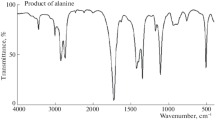
The kinetics of oxidation of l-glutamic acid (GLU) by diperiodatocuprate(III) (DPC) has been investigated in the presence of osmium(VIII) as homogeneous catalyst in alkaline medium at a constant ionic strength of 0.11 mol dm−3 spectrophotometrically. The reaction exhibits 1:4 stoichiometry ([GLU]:[DPC]). The order of the reaction with respect to [DPC] was unity, while the order with respect to [GLU] was less than unity over the concentration range studied. The rate was increased with an increase in [OH−] and decreased with an increase in [IO4 −]. The order with respect to [Os(VIII)] was unity. The ionic strength and dielectic constant of the medium did not affect the rate significantly. The main product succinic acid was identified by spot tests, FT-IR, and LC–MS spectral studies. Based on the experimental results, the possible mechanism was proposed. The reaction constants involved in the different steps of the mechanism were evaluated. The activation parameters with respect to the slow step of the mechanism were computed and also thermodynamic quantities determined. Kinetic studies suggest that the active species of DPC and Os(VIII) are found to be [Cu(H2IO6 )(H2O)2] and [OsO4(OH)2]2−, respectively.
Graphical abstract






Similar content being viewed by others
References
Reddy KB, Sethuram B, Navaneeth Rao T (1984) Indian J Chem 23A:593
Kumar A, Vaishali P, Ramamurthy P (2000) Int J Chem Kinet 32:286
Shettar RS, Nandibewoor ST (2005) J Mol Cat A Chem 234:137
Sethuram B (2003) Some aspects of electron transfer reactions involving organic molecules. Allied publishers (P) Ltd, New Delhi, p 78, p 151
Kitajima N, Moro-ka Y (1994) Chem Rev 94:737
Pierre JL (2000) Chem Soc Rev 29:251
Solomon EI, Chen P, Metz M, Lee SK, Palmer AE (2001) Angew Chem Int Ed 40:4570
Peisach J, Alsen P, Bloomberg WE (1996) The biochemistry of copper. Academic press, New York, p 49
Harihar AL, Panari RG, Nandibewoor ST (1999) Oxid Commun 22:308
Das AK (2001) Coord Chem Rev 213:307
Agarwal MC, Upadyay SK (1983) J Sci Ind Res 42:508
Veerasomaiah P, Reddy KB, Sethuram B, Navaneeth Rao T (1987) Indian J Chem 26A:402
Mohanty RK, Das M, Das AK (1997) Transit Met Chem 22:487
Sharma K, Mehrotra RN (2008) Polyhedron 27:3425
Puttaswamy, Shubha JP (2009) J Mol Catal A 310:24
Fiegl F (1975) Spot tests in organic analysis. Elsevier, New York, p 195
Jeffery GH, Bassett J, Mendham J, Denney RC (1996) Vogel’s text book of quantitative chemical analysis, 5th edn. England, ELBS Longman, Essex, p 381
Das AK, Das M (1994) J Chem Soc Dalton Trans 589
Lide DR (ed) (1992) CRC hand book of Chemistry and Physics, 73rd edn. CRC Press, London, p 8
Jagadeesh RV, Puttaswamy (2008) J Phys Org Chem 21:844
Reddy KB, Sethuram B, Navaneeth Rao T (1987) Z Phys Chem 268:706
Bailar JC Jr, Emeleus HJ, Nyholm SR, Trotman-Dikenson AF (1975) Comprehensive inorganic chemistry, vol 2. Pergamon Press, Oxford, p 1456
Angadi MA, Tuwar SM (2010) J Solut Chem 39:165
Kamble DL, Nandibewoor ST (1998) J Phys Org Chem 11:171
Lister MW (1953) Can J Chem 31:638
Cohen GL, Atkinson G (1964) Inorg Chem 3:1741
Naik KM, Nandibewoor ST (2012) J Chem Sci 124:809
Rangappa KS, Raghavendra MP, Mahadevappa DS, Channegouda D (1998) J Org Chem 63:531
Lewis ES (1974) Investigation of rates and mechanism of reactions. In: Weissberger A (ed) Techniques of chemistry, vol 6. Wiley, New York, p 421
Farokhi SA, Nandibewoor ST (2003) Tetrahedron 59:7595
Martinez M, Pitarque MA, Eldik RV (1996) J Soc Dalton Trans 2665
Murthy CP, Sethuram B, Navaneethrao T (1981) Z Phys Chem 262:336
Jeffery GH, Bassett J, Mendham J, Denney RC (1966) Vogel’s textbook of quantitative chemical analysis, 5th edn. UK, ELBS Longman, Essex, p 455
Panigrahi GP, Misro PK (1978) Indian J Chem 16A:201
Saxena OC (1967) Microchem J 12:609
Acknowledgments
One of the authors (PAM) thanks Department of Science and Technology, New Delhi for the award of INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
According to Scheme 2:
Total concentration of DPC is given by
where T and f refer to total and free concentrations. Therefore,
Similarly
In view of low concentration of Os(VIII) used, the term K 3[Os(VIII)] can be neglected. Hence
Similarly
The concentration of Os(VIII) is given by
Substituting Eqs. (9), (10), (11), and (12) in Eq. (8), we get
Rights and permissions
About this article
Cite this article
Magdum, P.A., Hegde, M.S., Singh, B.B. et al. Mechanistic aspects of osmium(VIII) catalysed oxidation of l-glutamic acid by copper(III) periodate complex in an aqueous alkaline medium. Monatsh Chem 147, 1703–1712 (2016). https://doi.org/10.1007/s00706-016-1672-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1672-3