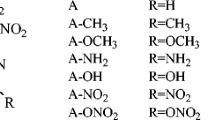Abstract
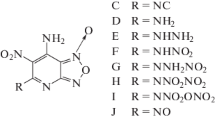
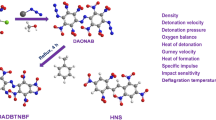
Using density functional theory calculations, we have investigated relationships between the structures and performance of a series of highly energetic diacetone diperoxides. The assigned infrared spectra of the compounds were used to compute the thermodynamic properties on the basis of the principle of statistical thermodynamics. The thermodynamic properties are linearly related with the number of –NNO2 groups as well as with temperature. The detonation pressures and velocities were evaluated using the Kamlet–Jacobs equations based on the theoretical density and condensed heat of formations. Results indicate that the replacement of H atoms of diacetone diperoxide by –NO2 groups is a better strategy for enhancing the detonation performance than replacement of O atoms by –NNO2 groups. It was found that by increasing the number of the nitro groups detonation properties will be increased. We suggest that octanitro-diacetone diperoxide may outperform the standard compound RDX (1,3,5-trinitro-1,3,5-trizinane), and will be a potential candidate for high-energetic density compounds.
Graphical abstract






Similar content being viewed by others
References
Chu F, Tsiminis G, Spooner NA, Monro TM (2014) Sens Actuator B Chem 199:22
Mastanaiah P, Madhusudhan Reddy G, Satya Prasad K, Murthy CVS (2014) J Mater Proc Tech 214:2316
Emich F (1900) Monatsh Chem 21:1061
Landenberger KB, Bolton O, Matzger AJ (2013) Angew Chem Int Ed 52:6468
Maty R, Pachman J (2010) Propellants Explos Pyrotech 35:31
Xu XJ, Xiao HM, Gong XD, Ju XH, Chen ZX (2005) J Phys Chem A 109:11268
Zhang J, Du H, Wang F, Gong X, Huang Y (2012) J Mol Model 18:165
Lin H, Chen P-Y, Zhu S-G, Zhang L, Peng X-H, Li K, Li H-Z (2013) J Mol Model 19:2413
Türker L, Atalar T, Gümüs S, Camur Y (2009) J Hazard Mater 167:440
Ochterski JW (2000) Thermochemistry in Gaussian. http://www.gaussian.com/thermo/thermo.pdf
Curtiss LA, Raghavachari K, Deutsch PW, Pople JA (1991) J Chem Phys 95:2433
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012) J Mol Model 18:2653
Chase MW, Davies CA Jr, Downey JR, Frurip DJ Jr, McDonald RA, Syverud AN (1985) J Phys Chem Ref Data 14(Suppl No 1)
Charlton MH, Docherty R, Hutchings MG (1995) J Chem Soc Perkin Trans 2:2023
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (1997) J Chem Phys 106:1063
Zhang XH, Yun ZH (1989) Explosive chemistry. National Defence Industry Press, Beijing
Qiu L, Xiao H, Gong X, Ju X, Jhu W (2007) J Hazard Mater 141:280
Zhao G, Lu M (2012) Bull Korean Chem Soc 33:1913
Pagoria PF, Lee JS, Mitchell AR, Schmidt RD (2002) Thermochim Acta 384:187
Harris NJ, Lammertsma K (1997) J Am Chem Soc 119:6583
Lide DR (ed) (2002) CRC handbook of chemistry and physics. CRC Press LLC, Boca Raton
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) J Mol Model 17:2569
Murray JS, Concha MC, Politzer P (2009) Mol Phys 107:89
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Scott AP, Radom L (1996) J Phys Chem 100:16502
Chen ZX, Xiao JM, Xiao HM, Chiu YN (1999) J Phys Chem A 103:8062
Beheshtian J, Peyghan AA, Bagheri Z (2012) Monatsh Chem 143:1623
Peyghan AA, Aslanzadeh SA (2014) Monatsh Chem 145:1253
Peyghan AA, Rastegar SF, Bagheri Z (2015) Monatsh Chem. doi:10.1007/s00706-014-1378-3
Peyghan AA, Soltani A, Pahlevani AA, Kanani Y, Khajeh S (2013) App Surf Sci 270:25
Beheshtian J, Peyghan AA, Bagheri Z (2012) J Mol Model 19:391
Rastegar SF, Peyghan AA, Hadipour NL (2012) App Surf Sci 265:412
Hill TL (1960) Introduction to statistic thermodynamics. Addison-Wesley, New York
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23
Chen ZX, Xiao JM, Xiao HM, Chiu YN (1999) J Phys Chem A 103:8062
Stine JR (1981) Los Alamos National Laboratory Report. New Mexico
Ammon HL (2001) Struct Chem 12:205
Karfunkel HR, Gdanitz RJ (1992) J Comput Chem 13:1171
Rice BM, Sorescu DC (2004) J Phys Chem B 108:17730
Qiu L, Xiao HM, Gong XD, Ju XH, Zhu W (2007) J Hazard Mater 141:280
Qiu L, Xiao HM, Ju XH, Gong XD (2005) Int J Quantum Chem 105:48
Qiu L, Xiao HM, Ju XH, Gong XD (2005) Chin J Chem Phys 18:541
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) Mol Phys 107:2095
Xu XJ, Xiao HM, Gong XD, Ju XH, Chen ZX (2005) J Phys Chem 109:11268
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahnooji, M., Pandas, H.M., Mirzaei, M. et al. Explosive properties of nanosized diacetone diperoxide and its nitro derivatives: a DFT study. Monatsh Chem 146, 1401–1408 (2015). https://doi.org/10.1007/s00706-015-1419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1419-6