Abstract
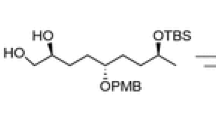
The total synthesis of 16-membered C 2-symmetric dilactone (−)-(5S,8R,13S,16R)-pyrenophorol was accomplished starting from enantiomerically pure propylene oxide prepared by hydrolytic kinetic resolution of (±)-propylene oxide with key steps of cross-metathesis and intermolecular Mitsunobu cyclization for the construction of macrolactone.
Graphical abstract


Similar content being viewed by others
References
Kis Z, Furger P, Sigg H (1969) Experientia 25:123
Grove JF (1971) J Chem Soc C:2261
Kind R, Zeeck A, Grabley S, Thiericke R, Zerlin M (1996) J Nat Prod 59:539
Kastanias MA, Chrysayi-Tokousbalides M (2000) Pest Manage Sci 56:227
Ghisalberti EL, Hargreaves JR, Skelton BW, White AH (2002) Aust J Chem 55:233
Krohn K, Farooq U, Flörke U, Schulz B, Draeger S, Pescitelli G, Salvadori P, Antus S, Kurtán T (2007) Eur J Org Chem:3206
Nozoe S, Hira K, Tsuda K, Ishibashi K, Grove JF (1965) Tetrahedron Lett:4675
Findlay JA, Li G, Miller JD, Womiloju TO (2003) Can J Chem 81:284
Grove TF, Saeake RN, Ward G (1996) J Chem Soc C:230
Powell J, Whalley WB (1969) J Chem Soc C:911
MacMillan J, Pryce RJ (1968) Tetrahedron Lett 9:5497
MacMillan J, Simpson TJ (1973) J Chem Soc Perkin Trans 1:1487
Ronald RC, Gurusiddaiah S (1980) Tetrahedron Lett 21:681
Christner C, Kullertz G, Fischer G, Zerlin M, Grabley S, Thiericke R, Taddei A, Zeeck A (1998) J Antibiot 51:368
Kibayashi C, Machinaga N (1993) Tetrahedron Lett 34:841
Zwanenburg B, Thijs L (1991) Tetrahedron Lett 32:1499
Yadav JS, Subba Reddy UV, Subba Reddy BV (2009) Tetrahedron Lett 50:5984
Oh H-S, Kang H-Y (2011) Bull Korean Chem Soc 32:2869
Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN (2002) J Am Chem Soc 124:1307
Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN (1997) Science 277:936
Louis JT, Nelson WL (1987) J Org Chem 52:1309
Kolb HC, VanNiewenhze MS, Sharpless KB (1994) Chem Rev 94:2483
Gerlach H, Gertle K, Thahnann A (1977) Helv Chim Acta 60:2860
Murthy IS, Sreenivasulu R, Alluraiah G, Raju RR (2014) Lett Org Chem 11:327
Acknowledgments
We are grateful to CSIR-IICT, Hyderabad for providing analytical facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Edukondalu, P., Sreenivasulu, R., Chiranjeevi, B. et al. Stereoselective total synthesis of (−)-(5S,8R,13S,16R)-pyrenophorol. Monatsh Chem 146, 1309–1314 (2015). https://doi.org/10.1007/s00706-014-1406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1406-3