Abstract
In order to improve efficiency, processability, and stability, two groups of novel poly(p-phenylene vinylene) (PPV) derivatives (P1–P3 and P4–P6) with hyperbranched structure and containing a nitro substituent were synthesized via a Gilch reaction in different monomer ratios. The properties of the polymers were investigated by using UV–Vis absorption, fluorescence spectroscopy, cyclic voltammetry, and thermogravimetric analysis. The result shows that the band gaps of the PPV derivatives with a nitro substituent were decreased and the polymers had higher molecular weights (106), excellent solubility in common organic solvents, good film-forming ability, and better thermal stability. The polymers can be used as an efficient acceptor material in polymeric solar cells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the discovery of electroluminescent poly(p-phenylene vinylene) (PPV) in 1990 [1], PPV continues to receive considerable interest for applications such as light-emitting diodes, field effect transistors, and photovoltaic devices owing to the polymer’s unique physical properties such as conductivity, electroluminescence, and solubility of selected derivatives [2–5]. PPV also encounters some problems, such as its tendency to aggregate, formation of excitation in the solid state leading to blue-green emission, fluorescence quenching, and the decrease in electroluminescence efficiency [6–8]. Although PPV is one of the best materials to be used as an efficient acceptor in polymeric solar cells (PSCs) because its structure causes low solubility and aggregation, the copolymers with other units which can overcome these problems have been investigated extensively.
Recently, hyperbranched polymers have drawn much attention and consideration owing to their ability to diminish the formation of interchain interactions owing to their high degree of branching, which can improve the solubility and thermal stability of PPV and reduce chain aggregation caused by fluorescence quenching. Considering these merits, we sought to synthesize a new kind of PPV which has hyperbranched structure [9–13].
Modified PPVs containing electron-withdrawing groups such as fluorine atoms, cyano groups, and heterocycles display high electron affinities (EA) and electron-transport properties as a result of increasing the polymer’s electron deficiency. The nitro moiety is a strong electron-withdrawing group, and inductive conjugation effects in nitrocellulose not only improve the nitro group’s electronic cloud density and increase the charge capacity, but also can enhance the electronic properties of conjugated polymers, increase the interaction between charges, and improve energy transfer and the quenching efficiency [14]. In order to reduce the band gap effectively, strong electron-withdrawing groups have been incorporated into the main skeleton to form a donor–acceptor (D–A) bridge.
Various modified Wessling reactions which originally use sulfur-based leaving groups have all been reported for the synthesis of a range of PPV derivatives [15–17]. This method has some drawbacks, such as the generation of toxic monomer during the solid-state elimination process, structural defects, and indefinable distribution of the molecular weights. The Gilch route is most widely used with long alkyl or alkoxy chains brought into the phenylene ring before polymerization to ensure the solubility [18, 19]. In this paper, we describe the synthesis and properties of a new family of hyperbranched copolymers. A novel “A2 + B3” approach based on Gilch polycondensation is used for the polymer synthesis.
In this publication, we report the synthesis and characterization of two groups of novel PPV derivatives (P1–P3 and P4–P6) with hyperbranched structure and containing a nitro substituent. Both groups of polymers exhibit a molecular weight of approximately 106, better solubility, a wide range of spectral absorption, and good thermal stability. These characteristics explain why the polymer is a good photoelectric material.
Results and discussion
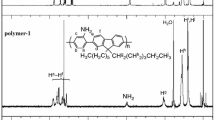
The detailed syntheses of the two groups of hyperbranched polymers are outlined in Scheme 1. The polymerization was done under different monomer feed ratios in tetrahydrofuran (THF) with freshly prepared potassium tert-butoxide. Polymers P1, P2, and P3 were obtained by reacting monomer A and 4 in molar ratios of 1.5:1, 4:1, and 9:1, respectively. Polymers P4, P5, and P6 were obtained by reacting monomer A and B in molar ratios of 3:1, 6:1, and 9:1, respectively. All polymers are soluble in common organic solvents, such as toluene, THF, chloroform, and methylene chloride. The molecular structures of the polymers were characterized with high-resolution NMR spectroscopy. 1H NMR revealed that the chemical shifts of the three symmetrical –CH2Br groups on the aryl of monomer 4 and B were about 4.45 ppm; those peaks disappeared and new vinylic proton peaks (at 7.45 ppm) and aromatic proton peaks appeared in the 1H NMR spectra of the polymers, suggesting that the polymerization was complete.

The number-average molecular weight (M n) and the weight-average molecular weight (M w) were determined by gel permeation chromatography (GPC) against standard polystyrene. The molecular weight values were merely estimates because of the differences in hydrodynamic radius between the hyperbranched polymers and the polystyrene standard. As shown in Table 1, the hyperbranched PPV derivatives prepared via Gilch reaction exhibit high molecular weights.
Thermal properties
The thermal properties of the hyperbranched polymers were investigated using thermogravimetric analysis (TGA). The polymers exhibited high thermal stability and onset of weight loss temperatures of 375, 377, 382 and 358, 379, 382 °C for P1, P2, P3 and P4, P5, P6, respectively, as shown in the TGA curves in Fig. 1. They were much higher than those for the linear MEH-PPV. The high thermal stability of these polymers could be of benefit to increase the stability of the PSC, which prevents morphological change, deformation, and degradation of the active layer [20].
Electrochemical properties
Cyclic voltammetry (CV) was employed to investigate the electrochemical properties of the six copolymers. The oxidation and reduction potentials revealed in cyclic voltammograms indicate the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels, which correspond to ionization potentials (IP) and EA, respectively [21]. The CV of polymer films cast from THF solution onto glassy carbon plate working electrodes, in 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6) in anhydrous acetonitrile as supporting electrolyte, were measured at a scanning rate of 10 mV/s.
The HOMO and LUMO energy levels of polymers were calculated and the onset potentials for oxidation and reduction are shown in Table 2. Firstly, without a nitro substituent (i.e., P1, P2, and P3), the onset potentials for oxidation (E oxonset ) were 1.39, 1.18, and 1.02 V and the onset potentials for reduction (E redonset ) were −1.67, −1.84, and −1.99 V. Secondly, with a nitro substituent (i.e., P4, P5, and P6), the onset potentials for oxidation (E oxonset ) were 0.99, 0.97, and 0.91 V and the onset potentials for reduction (E redonset ) were −1.80, −1.86, and −1.90 V.
The HOMO energy levels of the two groups of polymer were gradually reduced. The reason is possibly that the ratio of monomer A in these polymers increases little by little. The result indicates that hole-injection and transporting properties have been improved. The result implies that the electron-rich alkoxy groups affect the electronic properties of the conjugated copolymer chains significantly. Comparison of the two groups of polymers shows that the introduction of a nitro group in the hyperbranched PPVs not only increased the HOMO energy level, but also made the band gap narrower.
Photophysical properties
The photophysical characteristics of the polymers were investigated in solution and the thin film. The UV–Vis absorption and photoluminescence (PL) spectra of P1–P3 and P4–P6 in solution are shown in Figs. 2 and 3. Those for P1–P3 and P4–P6 in the film are shown in Figs. 4 and 5. The UV–Vis absorption maximum and PL emission maximum are summarized in Table 3. According to the spectra in Fig. 2, polymers P1–P3 exhibit only major absorption at 503, 493, and 490 nm in solution. In Fig. 3, P4–P6 show maximum absorptions at 497 nm in solution, and P4–P6 exhibit other major absorptions at 329, 333, and 335 nm in solution. Both groups of polymers absorb at around 500 nm, which is attributed to the transitions of main-chain π-systems of the polymer. The absorptions in the short wavelength region of the second group of polymers originate from the nitro substituent. The maximum absorption peak of polymers with a nitro substituent is sharper than that of polymers without this substituent. This is the reason why introducing the strong electron-withdrawing nitro group is good for the polymer π–π* interaction. The thin film absorption spectra show an obvious red shift relative to those in solution. According to the data given in Table 3, this red shift of the six polymers P1–P6 is 38, 29.5, 27, 8, 8, and 13 nm, respectively. The PL spectra were recorded with an excitation wavelength corresponding to the absorption maximum wavelength of the polymer. Emission peaks of P1, P2, and P3 in chloroform solution were all 567 nm, and in thin film were 602, 596, and 596 nm, respectively. Meanwhile, the emission peaks of P4, P5, and P6 in THF solution were all 553 nm, and in thin film were 581, 586, and 583 nm, respectively. This red shift could be explained by the enhancement of the interaction in the solid states.
Conclusion
We have successfully synthesized two groups of novel PPV derivatives (P1–P3 and P4–P6) with hyperbranched structure and containing a nitro substituent via the Gilch reaction. The introduction of trisubstituted benzene resulted in the twisting of the polymer backbone and thus decreased coplanarity. By introducing trisubstituted benzene with a nitro substituent, it increased the absorption range and lowered the polymer band gap. Poly-(1,3,5-tris(bromomethyl)benzene co 1,4-Bis(bromomethyl)-2-methoxy-5-(2’-ethyl)hexyloxybenzene phenylene vinylenene) (PTV-co-MEHPV) and Poly-(2-Nitro-l,3,5-tris(bromomethyl)benzene co 1,4-Bis(bromomethyl)-2-methoxy-5-(2’-ethyl)hexyloxybenzene phenylene vinylenene) (PTNV-co-MEHPV) have good solubility in common organic solvents. The number-average molecular weight and weight-average molecular weight are also large. All polymers we synthesized are red-light-emitting materials. The polymers can be used as an efficient acceptor material in PSC.
Experimental
1H NMR spectra of the compounds and polymers were recorded on a 400 MHz Varian Mercury NMR spectrometer in CDCl3 at room temperature, containing a small amount of TMS as internal standard. The molecular weights of the polymers were determined by GPC using a Perkin-Elmer series 200 apparatus in THF with polystyrene as standards. The flow rate of THF was maintained as 1.0 cm3/min at 40 °C. The thermal stability of the polymers was determined using a STA409 PC at a heating rate of 10 °C/min in a nitrogen atmosphere. The absorption and emission studies were done using a U-3310 UV–Vis spectrophotometer and a RF-5301P spectrofluorometer. The electrochemical measurements were performed using CV-50 W at a constant scan rate of 100 mV/s, with 0.1 M TBAPF6 solution in acetonitrile as the supporting electrolyte. A platinum wire and an Ag/AgNO3 electrode were used as the counter electrode and reference electrode, respectively. The solution spectra were recorded in THF and CHCl3 solutions, whereas the solid-state spectra were obtained from polymer thin films prepared by spin-coating the 10−3 mol/dm3 THF and CHCl3 solutions at 1,500 rpm on glass substrates.
Precursors 1–4 and monomers A and B were prepared according to literature procedures [22–24].
Polymer synthesis
A typical polymerization procedure for the preparation of the new polymers is described as follows. A solution of 8.4 cm3 of potassium tert-butoxide (2.0 M THF solution, 16.8 mmol) was slowly added over 1 h to a stirred solution of the two monomers at the chosen molar ratio (see below) in 25 cm3 dry THF that was cooled to 0 °C and under N2 atmosphere. The mixture was stirred at room temperature for 20 h. The polymerization solution was poured into 500 cm3 methanol. A crude polymer precipitated out, which was redissolved in chloroform and reprecipitated from methanol and then extracted in a Soxhlet apparatus with methanol to remove the impurities and oligomers. After drying filtration in vacuum, a bright red solid was obtained. In this paper, we adopt different ratios of starting monomers for the synthesis of the two groups of polymers. The proportions for P1–P3 are monomer A (0.2, 0.2, 0.1 mmol) and 4 (0.3, 0.8, 0.9 mmol); those for P4–P6 are monomer A (0.2, 0.2, 0.1 mmol) and monomer B (0.6, 1.2, 0.9 mmol). 1H NMR of P1 and P4 (400 MHz, CDCl3): δ = 7.54–7.09 (m, Ar–H), 4.14–3.85 (m, –OCH2–, –OCH3), 1.95–1.94 (m, –OCH2CH), 1.55–1.25 (m, –CH2), 0.89 (t, –CH3) ppm; 13C NMR of P1 (100 MHz, CDCl3): δ = 10.09, 13.99, 22.42, 30.50, 38.12, 39.56, 55.79, 65.29, 109.01, 124.40, 134.56, 144.22 ppm; 13C NMR of P4 (100 MHz, CDCl3): δ = 8.85, 17.44, 24.45, 32.22, 40.01, 51.73, 61.73, 110.76, 122.41, 137.91, 143.84, 145.51, 148.69 ppm.
References
Burroughes JH, Bradley DDC, Brown AR, Marks RN, Mackay K, Friend RH, Burns PL, Holmes AB (1990) Nature 347:539
Colladet K, Fourier S, Cleij TJ, Lutsen L, Gelan J, Vanderzande D, Huong Nguyen L, Neugebauer H, Sariciftci S, Aguirre A, Janssen G, Goovaerts E (2007) Macromolecules 40:65
Cho NS, Park JH, Lee SK, Lee J, Shim HK, Park MJ, Hwang DH, Jung BJ (2006) Macromolecules 39:177
Thompson BC, Kim YG, Reynolds JR (2005) Macromolecules 38:5359
Sun XB, Zhou YH, Wu WC, Liu YQ, Tian WJ, Yu G, Qiu WF, Chen SY, Zhu DB (2006) J Phys Chem B 110:7702
Chu HY, Hwang DH, Do LM, Chang JH, Shim HK, Holmes AB (1999) Synth Met 101:216
Hsieh BR, Yu Y, Forsythe EW, Schaaf GM, Feld WA (1998) J Am Chem Soc 120:231
Peng Z, Zhang J, Xu B (1999) Macromolecules 32:5162
Wooley KL, Frechet JMJ, Hawker CJ (1994) Polymer 35:4489
Shirota Y, Kuwabara Y, Inada H, Wakimoto T, Nakada H, Yonamoto Y, Kawai S, Imai K (1994) Appl Phys Lett 65:807
Wang KL, Huang ST, Hsieh LG, Huang GS (2008) Polymer 49:4087
Wang KL, Kakimoto M, Jikei M (2005) High Perform Polym 17:225
Wan WM, Pan CY (2008) Macromolecules 41:5085
Babudri F, Farinola GM, Naso F, Ragni R (2007) Chem Commun 1003
Louwet F, Vanderzande D, Gelan J, Mullens J (1995) Macromolecules 28:1330
Lutsen L, Adriaensens P, Becker H, Van Breemen AJ, Vanderzande D, Gelan J (1999) Macromolecules 32:6517
Son S, Dodabalapur A, Lovinger AJ, Galvin ME (1995) Science 26:376
Papadimitrakopoulos F, Konstadinidis K, Miller TM, Opila R, Chandross EA, Galvin ME (1994) Chem Mater 6:1563
Braun D, Heeger AJ (1991) Appl Phys Lett 58:1982
Tokito S, Tanaka H, Noda K, Okada A, Taga Y (1997) Appl Phys Lett 70:1929
Tsai LR, Chen Y (2008) Macromolecules 41:5098
Neef CJ, Ferraris JP (2000) Macromolecules 33:2311
Díez-Barra E, García-Martínez JC, Merino S, del Rey R, Rodríguez-López J, Sánchez-Verdú P, Tejeda J (2001) J Org Chem 66:5664
West AP Jr, Smyth N, Kraml CM, Ho DM, Pascal RA Jr (1993) J Org Chem 58:3502
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 61177029), the Science Foundation of the Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, Hubei University, Peoples’ Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Li, R., Mo, Y., Shi, R. et al. Synthesis and properties of poly(p-phenylene vinylene) derivatives with hyperbranched structure and containing a nitro substituent. Monatsh Chem 145, 85–90 (2014). https://doi.org/10.1007/s00706-013-1051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-1051-2