Abstract
A magnetic nanoparticle supported hyperbranched polyglycerol catalyst was prepared readily from inexpensive starting materials in aqueous medium that catalyzed the synthesis of 4H-benzo[b]pyran under solvent-free conditions at room temperature. X-ray diffraction, transmission electron microscopy, thermal gravimetric analysis, vibrating sample magnetometry, and selected-area electron diffraction were employed to characterize the properties of the synthesized catalyst. Its high catalytic activity and ease of recovery from the reaction mixture using an external magnet, and the possibility of reusing several times without significant loss of performance are additional eco-friendly attributes of this catalytic system.
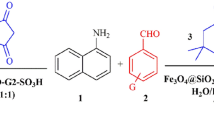
Graphical Abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, core–shell multi-components have attracted intense attention because of their potential applications in catalysis [1]. Unlike single-components that can supply only one function, core–shell multi-components can integrate multiple functions into one system for specific applications [2–6]. Moreover, the interactions between different components can greatly improve the performance of the multi-component system and even generate new synergetic properties. Among core–shell structured composites, those with a magnetic core and functional shell structures have received special attention because of their potential applications in catalysis, drug storage/release, selective separation, chromatography, and chemical or biologic sensors [7–12]. The magnetic core has good magnetic responsiveness, and can be easily magnetized. Therefore, composites with magnetic cores can be conveniently collected, separated, or fixed using an external magnet.
4H-Benzopyran derivatives are a major class of heterocycles, and 4H-pyran derivatives have attracted strong interest due to their useful biological and pharmacological properties such as anticoagulant, spasmolytic, diutretic, anticancer [13], and antianaphylactin characteristics [14]. 4H-Pyrans also occur in various natural products [15] and some benzopyran derivatives have been reported to have photochemical activities [16]. Development of 4H-pyran synthesis has been of considerable interest in organic synthesis, because of their wide-ranging biological and pharmaceutical activities. Consequently, numerous methods for the synthesis of 4H-pyrans have been reported. A variety of reagents, such as Yb(PFO)3 [17], tetramethylammonium hydroxide [18], Na2SeO4 [19], LiBr [20], NaBr [21], MgO [22], SB-DABCO [24], and the use of microwave irradiation [23], were found to catalyze these reactions. However, some of the reported methods have the following drawbacks: use of expensive reagents, long reaction times, low product yields, and use of an additional microwave oven. Herein we report the fabrication of hyperbranched polyglycerol (HPG) incorporated into mesoporous magnetite nanoparticles (MNP) that catalyze the synthesis 4H-benzo[b]pyrans under solvent-free conditions at room temperature (Scheme 1).

Results and discussion
We report the synthesis of a magnetic particle-based solid polymer with a high density of HPG groups and discuss its performance as a novel strong and stable solid polymer. We were intrigued by the possibility of applying anhydrous dioxane and nanotechnology to the design of a novel, active, recyclable, and magnetically recoverable HPG derivative for the first time (Fig. 1).
Normally, N2H4·H2O can serve as either an oxidant or a reducer in alkaline solution. Ni2+ can be reduced easily to Ni in alkaline solution by N2H4·H2O. However, it is difficult to reduce Fe2+ to Fe directly by N2H4·H2O because the electromotive force of the oxidation reaction of Fe2+ to Fe3+ (0.66 V) is much larger than that of the reduction reaction from Fe2+ to Fe (0.283 V). Thus, Fe2+ is more likely to be oxidized to Fe3+ when treated by N2H4·H2O in alkali solution. In this experiment, however, when Fe2+ and Ni2+ coexist in the solution, Fe2+ ions can be reduced easily to Fe under the assistance of Ni2+ to form FeNi3 alloy. The reduction reaction can be expressed as follows:
X-ray power diffraction
The structural properties of synthesized FeNi3/SiO2/HPG nanoparticle were analyzed by X-ray power diffraction (XRD). As shown in Fig. 2, the XRD pattern of the synthesized FeNi3/SiO2/HPG nanoparticle displays several relatively strong reflection peaks in the 2θ region of 40°–80°, which is quite similar to those of FeNi3 nanoparticles reported by other groups. Three characteristic peaks for FeNi3 (2θ = 44.3°, 51.5°, 75.9°) from (111), (200), and (220) planes were obtained. In addition, no iron and nickel oxides or other impurity phases were detected in the XRD patterns. The sharp and strong diffraction peaks confirm the good crystallization of the products. The broad band at 2θ = 15.0°–30.0° can be assigned to the amorphous SiO2 shell (JCPDS No. 29-0085).
High-resolution transmission electron microscopy
High-resolution transmission electron microscopy (HRTEM) images of FeNi3, FeNi3/SiO2, and FeNi3/SiO2/HPG MNPs are shown in Fig. 3. The average size of FeNi3 MNPs is about 15 nm, and the aggregation of the nanoparticles can be discerned clearly (Fig. 3a). After being coated with a silica layer, the typical core–shell structure of the FeNi3/SiO2 MNPs can be observed. The dispersity of FeNi3/SiO2 MNPs is also improved, and the average size increases to about 20 nm (Fig. 3b). The average size of FeNi3/SiO2/HPG MNPs is about 60 nm (Fig. 3c), but aggregation of FeNi3/SiO2/HPG is more evident than that of FeNi3/SiO2 MNPs.
Selected-area electron diffraction
The selected-area electron diffraction (SAED) pattern taken from the prepared FeNi3/SiO2/HPG MNPs consists of typical polycrystalline rings, suggesting a nanocrystalline structure (Fig. 4). The diffraction peaks from (111), (200), (220), and (311) planes of (FCC)-FeNi3 are in total agreement with those of XRD.
Thermogravimetric analysis
The thermal behavior of FeNi3/SiO2/HPG MNPs (Fig. 5) was evaluated to be 1.5 % according to thermogravimetric analysis (TGA). The analysis showed two decreasing peaks. The first peak appears at temperature around 130–150 °C due to desorption of water molecules from the catalyst surface. This is followed by a second peak at 425–450 °C, corresponding to the loss of the organic spacer group.
Magnetic properties of FeNi3/SiO2/HPG MNP
The magnetization curves of FeNi3 and FeNi3/SiO2/HPG MNPs were further recorded at room temperature (Fig. 6). The magnetizations were expressed in units of emu per gram of powder. The two measured samples display a superparamagnetic behavior, as evidenced by a zero coercivity and remanence on the magnetization loop. The saturation magnetization value of the FeNi3/SiO2/HPG MNP is 25 emu/g, which is lower than that of uncoated magnetic particles (about 60 emu/g).
Catalytic activity of FeNi3/SiO2/HPG MNPs
The effect of solvent on this reaction was examined and the results obtained are summarized in Table 1. In n-hexane, CHCl3, and dioxane (Table 1, entries 12–14), only a trace of product was observed. On the contrary, moderate yields could be achieved in other solvents (Table 1, entries 1–10). More strikingly, we found that the reaction proceeded smoothly in solvent-free conditions and gave the desired product in 97 % yield (Table 1, entry 11).
At this stage, the amount of catalyst necessary to promote the reaction efficiently was examined. It was observed that variation of the amount of FeNi3/SiO2/HPG MNP had an effective influence. The best amount of FeNi3/SiO2/HPG MNP was 0.001 g, which afforded the desired product in 97 % yield (Fig. 7).
Progress of the reaction in the presence of 0.001 g FeNi3/SiO2/HPG MNP was monitored by gas chromatography (GC) under optimal conditions (Fig. 8). Using this catalyst system, excellent yields of 4H-benzo[b]pyran can be achieved in 30 min. No apparent by-products were observed by GC in any of the experiments and the cyclic carbonate was obtained cleanly in 97 % yield.
It is important to note that the magnetic property of FeNi3/SiO2/HPG MNP facilitates its efficient recovery from the reaction mixture during work-up procedure. The activity of the recycled catalyst was also examined under the optimized conditions. After completion of the reaction, the catalyst was separated using an external magnet, washed with methanol and dried at the pump. The recovered catalyst was reused for eight consecutive cycles without any significant loss in catalytic activity (Fig. 9).
As can be seen from Table 2, the reaction of aromatic aldehydes with malononitrile and 1,3-diketones at room temperature under solvent-free conditions provided the corresponding 4H-benzo[b]pyran derivatives in good yields. The results presented in Table 2 indicate that aldehydes bearing electron-withdrawing groups react more quickly than their electron-donating aldehyde counterparts. For example, aromatic aldehydes such as 4-chloro-, 4-nitro-, and 4-bromobenzaldehydes react quickly with high product yields in comparison to 4-hydroxy-, 4-methyl-, and 4-methoxybenzaldehyde derivatives. The yield of 4H-benzo[b]pyrans bearing group at the ortho position on the aromatic ring is lower than that of the 4H-benzo[b]pyrans bearing group at the para position on the aromatic ring (Scheme 1; Table 2).
On the other hand, when benzyl cyanide was treated as a substitute for malononitrile in this reaction under similar conditions, not only was a highly prolonged time required, but the products were different. The spectroscopic data of the products confirmed that these structures belong to octahydroxanthene (Scheme 2).

In comparison with other catalysts employed for the synthesis of 4H-benzo[b]pyran from malononitrile, benzaldehyde, and dimedone, FeNi3/SiO2/HPG MNP showed a much higher catalytic activity in terms of a very much shorter reaction time and mild conditions (Table 3).
To further explore the potential of this MNP catalyst for heterocyclic synthesis, we investigated one-pot reactions involving aromatic aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate and obtained pyranopyrazoles in excellent yields (Scheme 3; Table 4). This methodology was evaluated using a variety of different substituted aromatic aldehydes in the presence of magnetic nanocatalyst under similar conditions. Aromatic aldehydes, carrying either electron-withdrawing or electron-donating substituents, afforded high yields of products with high purity; the results are presented in Table 4. The four-component cyclocondensation reaction proceeded smoothly and was completed in 35–50 min.

Conclusion
In conclusion, we have developed current important areas in the heterogenization of HPG—a rapidly developing research area. The main objectives are to develop room-temperature, solvent-free conditions, a rapid (immediate) and easy immobilization technique, and low-cost precursors for the preparation of highly active and stable MPs with high densities of functional groups. Furthermore, applying the exciting new area of magnetic particles that are intrinsically not magnetic, but can be magnetized readily by an external magnet, can have a positive effect on high activity on the one hand and separation and recycling on the other.
Experimental
Chemical materials were purchased from Fluka (Buchs, Switzerland) and Merck (Darmstadt, Germany) in high purity. Melting points were determined in open capillaries using an Electrothermal 9100 apparatus (http://www.electrothermal.com). Morphology was analyzed using high-resolution transmission electron microscopy (HRTEM) on a JEOL transmission electron microscope (http://www.jeol.com) operating at 200 kV. Powder X-ray diffraction data was obtained using Bruker D8 Advance model with Cu-Kα radiation. The thermogravimetric analysis (TGA) was carried out on a NETZSCH STA449F3 (http://www.netzsch-thermal-analysis.com) at a heating rate of 10 °C min−1 under nitrogen. The magnetic measurement was carried out in a vibrating sample magnetometer (VSM) (4 inch, Daghigh Meghnatis Kashan, Kashan, Iran) at room temperature. NMR spectra were recorded in DMSO-d 6 on a Bruker Avance DRX-400 MHz instrument spectrometer (http://www.bruker.com/) using tetramethylsilane (TMS) as internal standard. IR spectra were recorded on a Perkin Elmer 781 (http://www.perkinelmer.com/). Mass spectra were recorded on Shimadzu GCMS-QP5050 mass spectrometer (Shimadzu, Tokyo, Japan). The purity determination of the products and reaction monitoring were accomplished by thin layer chromatography (TLC) on silica gel polygram SILG/UV 254 plates.
Synthesis of FeNi3 MNPs
FeCl2·4H2O (1.72 g) and 4.72 g NiCl2·6H2O were dissolved in 80 cm3 deaerated highly purified water contained in a three-neck flask with vigorous stirring (800 rpm) under nitrogen. As the temperature was elevated to 80 °C, 10 cm3 ammonium hydroxide was added drop by drop, and the reaction was maintained for 30 min. The black product was separated by placing the vessel on a permanent magnet and the supernatant was decanted. The black precipitate was washed six times with highly purified water to remove unreacted chemicals, then the black product FeNi3 was dried under vacuum.
Synthesis of FeNi3/SiO2 MNPs
First, a mixture of 100 cm3 ethanol and 20 cm3 distilled water was added to 1 g magnetite nanoparticles, and the resulting dispersion was sonicated for 10 min. After adding 2.5 cm3 ammonia water, 2 cm3 tetraethyl orthosilicate (TEOS) was added to the reaction solution. The resulting dispersion was mechanically stirred continuously for 20 h at room temperature. The magnetic FeNi3/SiO2 nanoparticles were collected by magnetic separation and washed with ethanol and deionized water in sequence.
Synthesis of FeNi3/SiO2/HPG MNPs
For synthesis of FeNi3/SiO2/HPG MNPs, 2 mmol FeNi3/SiO2 MNPs were dispersed in a mixture of 80 cm3 toluene and 1.0 mmol potassium methanolate (CH3OK), followed by the addition of 10 cm3 anhydrous dioxane. Glycidol (2.0 g) was added dropwise over a period of 15 h. After vigorous stirring for 2 h, the final suspension was repeatedly washed, filtered several times, and air-dried at 60 °C.
General procedure for the synthesis of 4H-benzo[b]pyran
A mixture of aromatic aldehyde (1 mmol), dimedone (1 mmol), malononitrile (1 mmol), and 0.001 g FeNi3/SiO2/HPG MNP was stirred at room temperature under solvent-free conditions for the appropriate time (Table 2). Upon completion (the progress of the reaction was monitored by TLC), EtOH was added to the reaction mixture and the FeNi3/SiO2/HPG MNP was separated by external magnet. The solvent was then removed from solution under reduced pressure and the resulting product purified by recrystallization using ethanol.
General procedure for the synthesis of pyranopyrazoles
A mixture of ethyl acetoacetate (1 mmol), hydrazine hydrate (1 mmol), malononitrile (1 mmol), aldehyde (1 mmol), and 0.001 g FeNi3/SiO2/HPG MNPs was stirred at room temperature under solvent-free conditions for the appropriate time (Table 4). Upon completion (the progress of the reaction was monitored by TLC), EtOH was added to the reaction mixture and the FeNi3/SiO2/HPG MNPs was separated by external magnet. The solvent was then removed from solution under reduced pressure and the resulting product purified by recrystallization using ethanol.
References
Zhu CL, Zhang ML, Qiao YJ, Xiao G, Zhang F, Chen YJ (2010) J Phys Chem C 114:16229
Hu JQ, Bando Y, Zhan JH, Golberg D (2004) Appl Phys Lett 85:3593
Liu B, Zeng HC (2005) Small 1:566
Cao J, Sun JZ, Hong J, Li HY, Chen HZ, Wang M (2004) Adv Mater 16:84
Sun XM, Li YD (2004) Angew Chem Int Ed 43:597
Wang QB, Liu Y, Ke YG, Yan H (2008) Angew Chem Int Ed 47:316
Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME (2004) Nano Lett 4:719
Yang PP, Quan ZW, Hou ZY, Li CX, Kang XJ, Cheng ZY, Lin J (2009) Biomaterials 30:4786
Zhang M, Wu YP, Feng XZ, He XW, Chen LX, Zhang YK (2010) J Mater Chem 20:5835
Liu SS, Chen HM, Lu XH, Deng CH, Zhang XM, Yang PY (2010) Angew Chem Int Ed 49:7557
Won YH, Aboagye D, Jang HS, Jitianu A, Stanciu LA (2010) J Mater Chem 20:5030
Li Y, Wu JS, Qi DW, Xu XQ, Deng CH, Yang PY, Zhuang XM (2008) Chem Commun 2008:564
Foye WO (1991) Principal di Chemico Farmaceutica. Piccin, Padova, p 416
Singh K, Singh J, Singh H (1996) Tetrahedron 52:14273
Wang XS, Shi DQ, Tu ST, Yao CS (2003) Synth Commun 33:119
Armesto D, Horspool WM, Martin N, Ramos A, Seaone C (1989) J Org Chem 54:3069
Wang LM, Shao JH, Tian H, Wang YH, Liu B (2006) J Fluorine Chem 127:97
Balalaie S, Sheikh-Ahmadi M, Bararjanian M (2007) Catal Commun 8:1724
Hekmatshoar R, Majedi S, Bakhtiari K (2008) Catal Commun 9:307
Sun WB, Zhang P, Fan J, Chen SH, Zhang ZH (2010) Synth Commun 40:587
Elinson MN, Dorofeev AS, Feducovich SK, Gorbunov SV, Nasybullin RF, Miloserdov FM, Nikishin GI (2006) Eur J Org Chem 2006:4335
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) Eur J Med Chem 44:3805
Devi I, Bhuyan PJ (2004) Tetrahedron Lett 45:8625
Hasaninejada A, Shekouhya M, Golzara N, Zareb A, Doroodmand MM (2011) Appl Catal A 402:11
Fotouhi L, Heravi MM, Fatehi A, Bakhtiari K (2007) Tetrahedron Lett 48:5379
Hekmatshoar R, Majedi S, Bakhtiari K (2008) Catal Commun 9:307
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) Eur J Med Chem 44:3805
Rathod S, Arbad B, Lande M (2010) Chin J Catal 31:631
Sharanin YA, Sharanina LG, Puzanova VV (1983) J Org Chem USSR (Engl Transl) 1983:2291
Harb AA, Hesien AM, Metwally SA, Elnagdi MH (1989) Liebigs Ann Chem 1989:585
Abdel-Latif FF (1990) Z Naturforsch B: Chem Sci 45:1675
Sharanina LG, Promonenkov LG, Puzanova VV, Sharanona YA (1982) Chem Heterocycl Comp 18:607
Peng Y, Song G, Dou R (2006) Green Chem 8:573
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nasseri, M.A., Sadeghzadeh, S.M. Magnetic nanoparticle supported hyperbranched polyglycerol catalysts for synthesis of 4H-benzo[b]pyran. Monatsh Chem 144, 1551–1558 (2013). https://doi.org/10.1007/s00706-013-1026-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-1026-3