Abstract
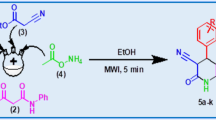
Twelve medicinally important pyrano[3,2-c]pyridine derivatives were precipitated, with high yields, from ethanol solutions of malononitrile and (E)-3,5-bis(benzylidene)-4-piperidones at ambient temperature, requiring almost no work-up. Natural bond order calculations at the B3LYP/6-31+G* level indicate that electron-withdrawing groups on the phenyl rings deplete electron density on β-atoms (with respect to the carbonyl groups) of the piperidones, leading to higher yields of the corresponding products with shorter reaction times. This green methodology appears in a clear contrast to all previous reports, where either a catalyst and/or microwave was employed. So, simplicity and short reaction time, non-toxicity of the solvent, as well as economic feasibility are major advantages of this chemically waste-free process.
Graphical abstract



Similar content being viewed by others
References
Mikami K (2005) Green reaction media in organic synthesis. Blackwell, Oxford
Lindstrom UM (2007) Organic reaction in water. Blackwell, Oxford
El-Subbagh HI, Abu-Zaid SM, Mahran MA, Badria FA, Al-Obaid AM (2000) J Med Chem 43:2915
Hammam AG, Sharaf MA, Abdel-Hafez NA (2001) Indian J Chem Sect B 40:213
Al-Omar MA, Youssef KM, El-Sherbeny MA, Awadalla AA, El-Subbagh HI (2005) Arch Pharm Chem 338:175
Rostom SAF, Hassan GS, El-Subbagh HI (2009) Arch Pharm Chem 342:584
Han Z-G, Tu S-J, Jiang B, Yan S, Zhang X-H, Wu S-S, Hao W-J, Cao X-D, Shi F, Zhang G, Ma N (2009) Synthesis 1639
Wang S-L, Han Z-G, Tu S-J, Zhang X-H, Yan S, Hao W-J, Shi F, Cao X-D, Wu S-S (2009) J Het Chem 46:828
Kumar RR, Perumal S, Senthilkumar P, Yogeeswarib P, Sriram D (2007) Bioorg Med Chem Lett 17:6459
Girgis AS, Ismail NSM, Farag H (2011) Eur J Med Chem 46:2397
Jin T-S, Liu L-B, Zhao Y, Li T-S (2005) Synth Commun 35:1859
Wang X-S, Shi D-Q, Du Y, Zhou Y, Tu S-J (2004) Synth Commun 34:1425
Zhou J-F (2003) Synth Commun 33:99
Rostamizadeh S, Azad M, Shadjou N, Hasanzadeh M (2012) Catal Commun 25:83
Rostamizadeh S, Amani AM, Mahdavinia GH, Sepehrian H, Ebrahimia S (2009) Synthesis 1356
Rostamizadeh S, Amirahmadi A, Shadjou N, Amani AM (2012) J Het Chem 49:111
Mahdavinia GH, Rostamizadeh S, Amani AM, Emdadi Z (2009) Ultrason Sonochem 16:7
Rui D, Daojun C, Yongjian Y (2011) Environ Toxicol Pharmacol 31:357
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG Jr, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millan JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelly C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98. Gaussian, Pittsburgh
Hariharan PC, Pople JA (1974) Mol Phys 27:209
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, Defrees DJ, Pople JA (1982) J Chem Phys 77:3654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) J Comput Chem 4:294
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265
Moller C, Plesset MS (1934) Phys Rev 46:618
Krishan R, Frisch MJ, Pople JA (1980) J Chem Phys 72:4244
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96:6796
Hu ZP, Lou CL, Wang JJ, Chen CX, Yan M (2011) J Org Chem 76:3797
Acknowledgments
The authors gratefully acknowledge the Research Council of K. N. Toosi University of Technology for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rostamizadeh, S., Kassaee, M.Z., Shadjou, N. et al. Efficient synthesis of pyrano[3,2-c]pyridines via a green and catalyst-free method at ambient temperature, and related DFT calculations. Monatsh Chem 144, 703–706 (2013). https://doi.org/10.1007/s00706-012-0851-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0851-0