Abstract
In the work discussed in this paper, cyclic voltammetric results obtained for the interaction of ascorbic acid, β-carotene, and three structurally related flavonoids (quercetin, rutin, and morin) with the anion radical of 1,3-dinitrobenzene were used to determine their antioxidant activity. The extent of the antioxidant–anion radical interactions was measured as the antioxidant activity coefficient. Higher values of this coefficient obtained for the three flavonoids in DMF are indicative of their greater antioxidant activity than ascorbic acid and β-carotene in this solvent. On the basis of cyclic voltammetric responses, a possible mechanism of the reaction of the reduction product of 1,3-dinitrobenzene with the antioxidants is proposed. Hyperchem PM3 quantum mechanical semi-empirical calculations of charges on reactive sites of the antioxidants and the 1,3-dinitrobenzene anion radical were also carried out; the results obtained supported the proposed mechanism of interaction.
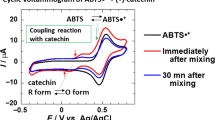
Graphical abstract




Similar content being viewed by others
References
Hermens JLM (1990) Environ Health Perspect 87:219
Carmichael PL, Stone EM, Grover PL, Gusterson BA, Phillips DH (1996) Carcinogenesis 17:1769
Grimmer G, Dettbarn G, Seidel A, Jacob J (2000) Sci Total Environ 247:81
Aßmann SN, Emmrich M, Kampf G, Kaiser M (1997) Mutat Res 395:139
Lissi E, Hanna MS, Pascual C, Castillo MDD (1995) Free Radic Biol Med 18:153
Halliwell B (1996) Annu Rev Nutr 16:33
Pedrielli P, Skibsted LH (2002) J Agric Food Chem 50:7138
Peng IW, Kuo SM (2003) J Nutr 133:2184
Havsteen BH (2002) Pharmacol Therapeut 96:67
Silva MM, Santos MR, Caroco G, Rocha R, Justino G, Mira L (2002) Free Radic Res 36:1219
Husain SR, Cillard J, Cillard P (1987) Phytochem 26:2489
Seyoum A, Asres K, El-Fiky FK (2006) Phytochem 67:2058
de Souza RFV, Sussuchi EM, de Giovani WF (2003) Synth React Inorg Met–Org Chem 33:1125
Arshad N, Janjua NK, Ahmed S, Khan AY, Skibsted LH (2009) Electrochim Acta 54:6184
Feroci G, Fini A (2007) Inorg Chim Acta 360:1023
Korotkova EI, Avramchik OA, Kagiya TV, Karbainov YA, Tcherdyntseva NV (2004) Talanta 63:729
Ziyatdinova GK, Gil’metdinova DM, Budnikov GK (2005) J Anal Chem 60:49
Pinelo M, Manzocco L, Nuñez MJ, Nicoli MC (2004) Food Chem 88:201
Pedrielli P, Pedulli GF, Skibsted LH (2001) J Agric Food Chem 49:3034
Liu H, Xiang B, Qu L (2006) J Mol Struct 794:12
Denisov ET, Afenas’ev IB (2005) Oxidation and antioxidants in organic chemistry and biology. Taylor & Francis. CRC Press, Boca Raton
Acknowledgments
The authors acknowledge the Higher Education Commission (HEC) of Pakistan for financial support. We also highly acknowledge Dr Uzma Yunus, Dr Zaman Asraf, Ms Azra Yaqub, and Mr Sheikh Faisal Azeem for their valuable suggestions and needful help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arshad, N., Janjua, N.K., Khan, A.Y. et al. Electrochemical studies of the interactional mechanism and scavenging activity of antioxidants towards dinitroaromatics. Monatsh Chem 143, 377–383 (2012). https://doi.org/10.1007/s00706-011-0606-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0606-3