Abstract
The International Union of Pure and Applied Chemistry (IUPAC) project of collection, compilation, and critical evaluation of solubility data of bromides and iodides of the scandium group and all lanthanides in water and aqueous systems containing either halide acids, halide salts, or organic compounds is under preparation. As a result of their similarity to the chlorides, which were recently evaluated, the bromides and iodides in the lanthanide series should show some regularities in their solubility data. Unfortunately, the corresponding results show a large scatter when ordered according to the atomic number. Thus, it is complicated to select the best data for recommendation. Reasons for the inaccuracy of solubility measurements are outlined. In fact some solubility values of bromides predicted by correlation with chlorides seem to be more reliable than the experimental ones. As sufficient experimental data at various temperatures were available, the water-rich fragment of the LaBr3–H2O equilibrium phase diagram has been formed and depicted. It seems to be similar to the well-known LaCl3–H2O diagram. Several regularities, with respect to stoichiometry and solubility of compounds formed, were observed during investigations of the aqueous ternary systems. The complex iodides of various lanthanides display more regularities in their properties than the bromides do.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This is a brief assessment which is a continuation of the critical evaluation of solubilities of rare earth metal halides in water-containing systems. The part on solubilities of chlorides was already published [1–3]. It would seem that bromides and iodides of rare earth metals are less significant than chlorides. However, running through Chemical Abstracts one may find plenty of contemporarily important applications of these compounds in technology and science: for production of alloys and compounds, as catalysts in organic synthesis and polymerization, as corrosion inhibitors, as converting materials for various kinds of spectroscopy, as lighting and laser materials, as solid electrolytes for lithium batteries, and as dyes for glass, ceramics, and ink. Investigation of the solubility equilibria in ternary and multicomponent systems is useful for the detection of eventual formation of double salts, complex compounds, and solid solutions of the hydrates. The knowledge about such systems may improve the extraction and refining processes of rare earth metals.
Solubility data for rare earth metal bromides and iodides in water
Although a number of solubility studies of rare earth metal bromides and iodides were published, there is no systematic critical evaluation of these results so far. The first step of such a procedure after the data collection is an estimation of experimental precision and accuracy. Unfortunately, this information was seldom available in the papers. In the case of bromides, the laboratories of Nishimura et al. [4] and Voigt [5] achieved precision in some experiments of ±0.1% and in the laboratory of Qiao et al. [6] it was specified as ±0.2%. The rest of the laboratories did not report any uncertainties and we estimate that the precision was then ±(1–5)%. In the case of iodides, the laboratory of Alikberova specified the precision as ±0.2% [7] or even ±0.05% [8], but in this case we prefer a less optimistic estimate of ±0.5%, and we appraise that precision in other laboratories, which did not specify it, was ±(1–5)%.
We selected the most reliable values from the existing solubility data collection [9] for the bromides and iodides and they are presented in Fig. 1. For comparison, Fig. 1 also contains the recommended values of the solubility of rare earth metal chlorides at 298.2 K [1–3]. These values are precisely known within ±0.1%, and therefore they may be used as the reference data basis.
If one intends to follow the solubility changes with regard to the atomic number (or ionic radius) of the rare earth metal one should compare the solute salts which possess the same stoichiometry and crystal structure of its hydrate. Concerning the chlorides one observes two parabola-like curves: the first one being placed between La and Pr chlorides when the equilibrium solid phase is isostructural triclinic LnCl3·7H2O and the second one extending between Nd and Lu (including also Y) when the equilibrium solid phase is isostructural monoclinic LnCl3·6H2O [1]. As a result of their similarity to the chlorides, the bromides form the isostructural triclinic LnBr3·7H2O phase for La, Ce, and Pr and the isostructural monoclinic LnBr3·6H2O phase for lanthanides between Nd and Yb. However, there are contradictory opinions about the stoichiometry of the equilibrium solid phase for the range Ho to Lu; it is either the isostructural monoclinic LnBr3·6H2O or monoclinic LnBr3·8H2O phase [9–12]. In the case of iodides, isostructural orthorhombic LnI3·9H2O was identified for the lanthanide range of La to Er and isostructural monoclinic LnI3·8H2O for the range of Tm to Lu [10]; however, different ranges of the solid solute existence were reported [11, 12]. It is a well-known fact that structures and stoichiometries of YBr3 and YI3 hydrates resemble those typical for lanthanides, but crystals of ScBr3 and ScI3 hydrates are dissimilar compared with the corresponding lanthanide compounds.
The segments of the curve for chlorides in Fig. 1 are quite smooth; the solubility changes gradually reach the minimum at TbCl3. In the case of bromides the scatter of the selected solubilities is significant (up to 20%). Thus, the expected similarity between the chloride and bromide’s solubility dependence on the atomic number is blurred. Because the mean molal activity coefficients of concentrated solutions [13], the enthalpies of formation of the hydrates, and their dehydration and lattice energies for the isostructural bromides as well as chlorides change smoothly with the rare earth metal atomic number [14], the reliable solubility results for the bromides should form a parallel-like curve moved a fraction up in comparison to the chlorides’ curve. On the basis of this assumption, we feel empowered to predict the solubilities of the bromides more reliably than they were determined. The predicted solubilities for the bromides from Nd to Yb, which form hexahydrates, at about 300 K, are collected in Table 1.
The predicted values were obtained by the addition of Δx = 0.009 to the recommended mol fraction values for chlorides [1–3]; the value 0.009 is the mean difference of the solubility data between all bromides and chlorides. Thus, the parabola-like curve for the chlorides has been moved up by Δx = 0.009 on the mol fraction scale to predict the solubility of bromides. We are convinced that our predictive values are more reliable than the very scattered experimental data. As was shown in the case of LaBr3 the temperature dependence of the solubility near 300 K is very small, about 0.0002 in mol fraction per K; therefore the difference of temperatures in Table 1 is not essential in such predictions. If in the range Ho to Yb the equilibrium solid phase at about 300 K is of LnBr3·8H2O type then the predicted solubility values for the bromides of these elements in Table 1 will be invalid. A similar procedure may be proposed for the bromides of La–Pr which form heptahydrates; however, the only reliable value of the solubility is known for LaBr3.
Because most of the chlorides and iodides form different crystal structures and there are less thermodynamic data for the iodides (the enthalpies of formation and dissolution of LnI3 in water both exhibit a smooth dependence on the atomic number with a small break at Gd [15]) a similar prediction procedure for the iodide solubilities would be premature; however, the solubility changes in the rare earth metal iodides row between La and Eu should be distinctly more smooth than that experimentally observed, and probably the solubility minimum value for EuI3 is fortuitous.
It seems that there are several causes of the uncertainties of the solubility data. The equilibria between solids and aqueous solutions in these systems are reached after a very long time. This phenomenon proceeds along with a strong tendency toward oversaturation. The solubilities of LnBr3 and LnI3 depend explicitly on pH in the saturated solutions which is exemplified best by the investigation of the Ho2O3 solubility in HI solution [16]. When Ho2O3 and HI were taken in stoichiometric ratio (1:6) to form HoI3 then solubility of this salt reached the highest value. When Ho2O3 was in excess then the less soluble Ho2I(OH)5 salt was formed, this may be interpreted as the following hydrolysis reaction:
When HI was in excess relative to Ho2O3 then the solubility of HoI3 clearly decreased, presumably due to the common ion effect. Therefore, the proper pH of the solution and the correct stoichiometry of the salt LnI3 should be strictly controlled because only then is its solubility the highest. Complexation of the rare earth ions with an excess of either Br− or I−, separately, is rather weak in the corresponding solutions [17]; however, as was shown during the investigations of ternary systems with other halide salts, double salts may be formed in the corresponding ternary systems. It is sure that the investigations of the iodides must be performed in an oxygen-free atmosphere to avoid decomposition of the iodides and subsequent iodine evolution. Hence, the solubility measurements of the iodides were preferably performed at 273 K and quite seldom at higher temperatures. Likewise, during careful observations of the bromides during the experiments we have noticed a light bronzing of the samples which may mean that the analogous oxidation of bromides may also occur. There are the special cases of EuI3 and YbI3 which due to the dismutation-like reactions form quite stable salts in their divalent state with an evolution of either Br2 or I2, probably also influencing the solubility determinations. Unfortunately, the solubilities of the divalent bromides and iodides were not determined but these salts also seem to have good solubility.
It appears that the method of experimental determination of solubility plays a secondary role in the uncertainty of the results because, quite frequently, the values obtained in one laboratory also showed drastic differences for analogous systems of the neighboring rare earth elements. The following methods of analysis of the saturated solutions have been used: titration of Ln ions with EDTA solution, spectrophotometric determination in the case of simultaneous analysis of two Ln metals, precipitation of Ln oxalate with ignition to Ln2O3 and weighing, an oxidimetric titration in the case of Ce, argentometric titration of Br or I ions (sometimes with the potentiometric detection of equivalent point), and precipitation of AgI and weighing. Exceptionally, the isopiestic technique was used in the case of ScBr3 solubility determination and some differential scanning calorimetry (DSC) experiments were performed for LaBr3 [9]. If either the Ln or the Br (or I) content was analyzed only whereas the stoichiometry of a salt was not precisely checked then the possibility of erroneousness of the solubility determination increased.
When the solubility experiments are extended to higher temperatures then one will be faced with some experimental difficulties due to the hydrolysis reaction written schematically for bromides:
The use of a high pressure and acid-resistant apparatus is indispensable under such experimental conditions. Because the undesirable reaction (2) is a moderately fast process it is suggested to perform a short thermal analysis run rather than an extended solubility equilibration.
Probably due to the mentioned difficulties, the solubility determinations at higher temperatures were only effectively performed for the LaBr3–H2O system with the use of chemical analysis of the equilibrated phases as well as some complementary DSC measurements [5]. The solubility results obtained for this system between 298 and 393 K allowed one to formulate the proper solubility equation. As was observed in the case of many chloride systems [1–3], the experimental values of LaBr3 solubility in water collected in Table 2 do not fit, and they should not fit, to the simple linear equation:
which can be valid for non-electrolyte solutions. On the left-hand side of Eq. 3, the ionic mol fractions [18] should be taken into account.
Therefore, the following equation, used by the IUPAC Subcommittee on Solubility and Equilibrium Data [19, 20], was taken into account:
where x 1 is the solubility of the salt expressed in mol fractions, T the absolute temperature in K, v the number of ions produced upon salt dissociation, r the number of solvent molecules in the formula of the solid solvate equilibrated. The constants A, B, C, and D can be derived from a fitting procedure if more than four solubility results are at our disposal, and r and v are known from other experiments. Thus, the following solubility equation was obtained by fitting to the solubility data (from Table 2) for LaBr3·7H2O in the temperature range 298–389.6 K:
In other systems, the corresponding solubility data were either not reliable (as for the YBr3–H2O) or a considerable part of the data related to different equilibrium phases (as for the LaI3–H2O system) and then Eq. 4 loses its validity.
Phase diagrams at the water-rich side
Concerning the composition of equilibrium phases at higher temperatures, the thermogravimetric technique in connection with differential thermal analysis (DTA) (or DSC) was found to be very informative. It was well proved for LaBr3·7H2O that with an increase of temperature the following dehydration stages were observed:
This behavior is distinctly reflected in the experimental run shown in Fig. 2. The similar dehydration scheme may be also proposed for CeBr3·7H2O and PrBr3·7H2O.
TG thermogravimetric [as relative mass decrement, (m1 − m2)/m1], TDG differential thermogravimetric [as mass decrement in time, (Δm/Δt)], and DTA differential thermal analysis (as voltage difference between thermocouple legs, V) curves of LaBr3·7H2O. The experiment was performed on a DTA/TG SSC 5200 (Seiko Instruments), with a heating rate 5 K/min, in a Pt crucible, in Ar atmosphere
The observed scheme for the bromide hexahydrates of heavier lanthanides was simpler, as it is exemplarily shown for SmBr3·6H2O:
However, as a result of its similarity to the chlorides [1–3], the bromide should also be able to form SmBr3·3H2O at a slower heating rate than that applied by Mayer and Zolotov [21]. Consequently, one may predict that the phase diagrams of other LnBr3–H2O systems should be similar in the form of the liquidus shape, composition, and stability of the equilibrium solid phases (types of the hydrates).
Likewise, the lighter Ln iodides dehydrate gradually according to the following reaction chain:
whereas DyI3·9H2O, HoI3·9H2O, ErI3·8H2O, TmI3·8H2O, YbI3·8H2O, and LuI3·8H2O decompose to the anhydrous LnI3 salts in one step according to Heiniö et al. [22]. Crystallographic studies of the hydrates confirmed that they were isostructural: monoclinic P21/n for LnBr3·8H2O (Ho–Lu), triclinic \(P\bar 1\) for LnBr3·7H2O (La–Pr), monoclinic P2/n for LnBr3·6H2O (Nd–Dy), orthorhombic Pmma for LnI3·9H2O (La–Lu), unindexed (probably monoclinic) for LnI3·8H2O (Tm–Lu), monoclinic P2/n for LnI3·6H2O (La–Tb), and unindexed for LnI3·3H2O (La–Nd) [10, 12, 22–24]. This detailed information is useful for calculation and depiction of the partial LnBr3–H2O and LnI3–H2O phase diagrams. Analogous Y compounds have the typical structures of other heavy Ln halides; however, Sc compounds are different.
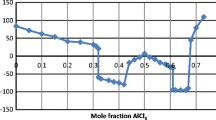
In the case of chlorides, it was possible to draw many of the LnCl3–H2O phase diagrams on the water-rich side. However, in this case, there were sufficient data to construct only the part of the LaBr3–H2O phase diagram depicted in Fig. 3. We are convinced that the rest of the LaBr3–H2O phase diagram is qualitatively similar to the well-known LaCl3–H2O phase diagram [1].
For the design of the LaBr3–H2O equilibrium phase diagram we used the results of the thermogravimetric, DTA, crystallographic, and solubility studies in the extensive temperature range 298–393 K which were supplemented by DSC experiments at lower temperatures. Temperature of the congruently melting LaBr3·7H2O was determined to be 389.6 ± 0.5 K. An example of the DSC run is shown in Fig. 4.
One may easily see that both very asymmetrical exothermic and endothermic peaks, being distant for almost 20 K, are in fact related to the same phase transition because the heat exchanged during the crystallization and melting of LaBr3 is practically the same. The very sharp exothermic peak informs us that the crystallization in this system occurs with a significant undercooling effect. This is an additional argument explaining why the experiments with the presented systems are experimentally so difficult. We are continuing investigations at compositions of LaBr3 lower than 0.07 mol fraction to get more complete information about the water-rich part of this phase diagram. Unfortunately, the phase diagrams of other bromides and iodides still await construction due to the lack of sufficient solubility data at various temperatures, a precise estimation of the composition of the hydrates, and their decomposition temperatures.
Ternary and quaternary aqueous systems
Many experimental papers were devoted to the aqueous ternary systems of LnBr3 or LnI3 with inorganic and organic compounds as third components. As we mentioned earlier, the solubilities of the majority of bromides or iodides being investigated decreased upon addition of either HBr or HI. This phenomenon may be treated as the rule. Table 3 shows an example of the decreasing solubility of LuI3 with increasing content of HI in the solution; the decrease measured was substantial.
Investigations on the ternary systems of LnBr3 with KBr showed that they display a eutonic-type equilibrium, whereas double salts of general formula 2LnBr3·5CsBr·nH2O were formed with CsBr; the double salts were also formed in the presence of HBr as the fourth component. Neither NaI, KI, NH4I, nor RbI formed double salts with LnI3 in the aqueous ternary systems. When I2 was present in LnI3 solutions then polyiodide ions (I3 −, I5 −) were detected in respective solutions; however, a crystallization of the corresponding polyiodide salts was not observed due to their high solubility [25].
A complete solid miscibility of LaCl3 and LaBr3 heptahydrates was found in the LaCl3–LaBr3–H2O system. In the investigations of some LnI3–Ln′I3–H2O systems, a good solid miscibility of both LnI3·9H2O and Ln′I3·9H2O was observed when differences of the ionic radii of Ln and Ln′ were smaller than 5%; for larger differences a limited solid miscibility was observed with sometimes unexplained exclusions [9].
The bromides formed complexes with various organic compounds [9]. Glycine (C2H5NO2) formed a series of complexes of the formula LnBr3·3C2H5NO2·3H2O. Interaction of LnBr3 with urea and its derivatives (thiourea, acetamide, biuret, acetylurea) was investigated unsystematically; however, some general observations may be formulated. Acetamide, biuret, and acetylurea showed affinity to the bromides resulting in a precipitation of complex compounds, whereas no complexes were identified with thiourea and such systems were of eutonic type. If a complex formation was found with one lanthanide bromide then its analogues were also found for other lanthanide bromides; nevertheless the stoichiometry of such compounds may be changed in the series.
The systems of organic compounds with the iodides [9] were more intensively investigated than with the bromides. All lanthanide iodides formed a complex with urea (CH4N2O) of general formula LnI3·5CH4N2O and this complex with the heavy lanthanides displayed almost constant solubility of 5.6 ± 0.1 mol kg−1, whereas for the light lanthanides the solubility values were scattered irregularly between 4.7 and 8.0 mol kg−1. An additional presence of I2 in the system led to the formation of LnI3·4I2·5CH4N2O·10H2O (La–Tm) or LuI3·3I2·5CH4N2O·7H2O [25]. Thiourea (CH4N2S) formed the LnI3·2CH4N2S·10H2O series of compounds and the pertinent solubilities measured were scattered between 3.5 and 6.0 mol kg−1, but many of these solubility values were estimated from small figures and may therefore be accurate to only ±0.5 mol kg−1. Urotropine hydriodide (C6H12N4·HI) formed LnI3·C6H12N4·HI·14H2O complexes and biuret (C2H5N3O2) formed ErI3·4C2H5N3O2; the corresponding solubility results were presented only graphically and could not be precisely evaluated. A quite smooth dependence of solubility ordered according to the atomic number of the lanthanide was displayed by the complexes with antipyrine (C11H12N2O) of general formula LnI3·6C11H12N2O; the corresponding solubility results are presented in Table 4.
Quite unexpectedly, for these complex compounds we observe much more regular changes of the solubilities than were observed for the simple bromides and iodides.
References
Mioduski T, Gumiński C, Zeng D (2008) J Phys Chem Ref Data 37:1765
Mioduski T, Gumiński C, Zeng D (2009) J Phys Chem Ref Data 38:441
Mioduski T, Gumiński C, Zeng D (2009) J Phys Chem Ref Data 38:925
Nishimura Y, Iwamoto K, Suzuki Y (1994) Abstracts of the 17th symposium on solution chemistry, Okayama, Japan, p 138
Voigt H (1994) Rep. no Vo 511/1-2, Inst Anorg Chem, Bergakademie, Freiberg, Germany (to be published)
ZhP Qiao, Chen JG, Zhuo LH, ShSh Zhang (2008) Zh Neorg Khim 53:498
Alikberova LYu, Rukk NS, Li V, Stepin BD (1991) Zh Neorg Khim 36:3110
LYu Alikberova, AYu Tsitrina, Yastrebova LF, Stepin BD (1974) Zh Neorg Khim 19:284
Mioduski T, Gumiński C, Voigt H, Zeng D (2011) J Phys Chem Ref Data (to be published)
Boyle TJ, Ottley LAM, Alam TM, Rodriguez MA, Yang P, McIntyre SK (2010) Polyhedron 29:1784
Burgess J, Kijowski J (1981) Adv Inorg Chem Radiochem 24:57
Lim KC, Skelton BW, White AH (2000) Aust J Chem 53:867
Fukushi K, Iwamoto K, Kobayashi K, Ishikawa K, Suzuki Y (1994) J Alloys Compd 207/208:468
TSh Sun, Wang FL, Xiao YM (1998) Thermochim Acta 311:21
Hohmann E, Bommer H (1941) Z Anorg Chem 248:383
Danilenko TV, Emelyanov VI, Venskovskii NU, Ezhov AI (1998) Zh Neorg Khim 43:139
Sillen LG, Martell A (1964 and 1971) Stability constants, special publication nos 17 and 25. The Chemical Society, London
Gamsjäger H, Lorimer JW, Scharlin P, Shaw DG (2008) Pure Appl Chem 80:233. http://www.iupac.org/publications/pac
Lorimer JW, Cohen-Adad R (2003) Thermodynamics of solubility. In: Hefter GT, Tomkins RPT (eds) Experimental determinations of solubilities, chap 1.1. Wiley, Chichester, p 55
Gamsjäger H, Lorimer JW, Salomon M, Shaw DG, Tomkins RPT (2010) Pure Appl Chem 82:1137
Mayer I, Zolotov S (1965) J Inorg Nucl Chem 27:1905
Heiniö O, Leskelä M, Niinistö L (1980) Acta Chim Scand Ser A 34:207
Kwestroo W, Van Hal HAM (1976) J Inorg Nucl Chem 38:1019
Junk PC, Semenova LI, Skelton BW, White AH (1999) Aust J Chem 52:531
Rukk NS, Savinkina EV, Kravchenko VV, Alikberova DV, Yu L (1997) Russ J Coordin Chem 23:208; Koordin Khim 23:226
Alieva ZF, Sulaimankulov K (1981) Spravochnik po Rastvorimosti Karbamidnykh Solevykh Sistem. Mekter, Frunze, p 232
Zwietasch KJ, Kirmse EM, Krech I (1984) Z Chem 24:144
Zholalieva ZM, Sulaimankulov KS, Ismailov M (1975) Zh Neorg Khim 20:2005
Zholalieva ZM, Sulaimankulov K, Ismailov M (1977) Fiziko-Khimicheskie Izucheniya Soedinenii Metallov z Biologichesko Aktivnymi Veshchestvami. Akademiya Nauk Kirgizkoi SSR, Frunze, p 90
Acknowledgments
This publication was partly based on the literature collected by the late Tomasz Mioduski. The authors are indebted to the IUPAC Subcommittee of Solubility and Equilibrium Data for encouragement during the project preparation. C. G. thanks the University of Warsaw for the financial support (project BST-143131).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gumiński, C., Voigt, H. & Zeng, D. Solubility of rare earth metal bromides and iodides in aqueous systems. Monatsh Chem 142, 211–218 (2011). https://doi.org/10.1007/s00706-011-0457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0457-y