Summary.
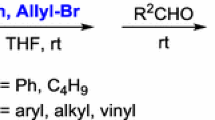
The successful Pd-catalysed allylation of 2-phenylpropanal with allyl alcohol in ionic liquid is described and thus a simplier reaction composition catalytic system than in THF is possible. On the other hand, the Pd-catalyzed α-allylation of the same substrate with allyl acetate or allyl ethyl carbonate is proceeding nicely in ionic liquids. Allylation of different carbonyl derivatives was studied and it was found that the reaction is restricted to carbonyl derivatives from which a carbanion stabilized by an adjacent aromatic ring can be formed.
Similar content being viewed by others
References
Godkleski SA (1991) Comprehensive Organic Synthesis. In: Trost BM, Fleming I, Paquette LA (eds), vol. 4. Pergamon Press, Oxford, p 585
G Consiglio RM Waymouth (1989) Chem Rev 89 257 Occurrence Handle10.1021/cr00091a007 Occurrence Handle1:CAS:528:DyaL1MXnsVCgug%3D%3D
BM Trost DL Van Varken (1996) Chem Rev 96 395 Occurrence Handle10.1021/cr9409804 Occurrence Handle1:CAS:528:DyaK28XjsVOiuw%3D%3D
J Tsuji I Minami (1987) Acc Chem Res 20 140 Occurrence Handle10.1021/ar00136a003 Occurrence Handle1:CAS:528:DyaL2sXhvFGrs70%3D
BM Trost (1980) Acc Chem Res 13 385 Occurrence Handle10.1021/ar50155a001 Occurrence Handle1:CAS:528:DyaL3cXlvVGhsro%3D
BM Trost (1989) Angew Chem Int Ed Engl 28 1173 Occurrence Handle10.1002/anie.198911731
BM Trost C Lee (2000) NoChapterTitle I Ojima (Eds) Catalytic Asymmetric Synthesis Wiley-VCh New York
BM Trost L Weber PE Strenge TJ Fulleron TS Dietsche (1978) J Am Chem Soc 100 3426 Occurrence Handle10.1021/ja00479a026 Occurrence Handle1:CAS:528:DyaE1cXlt12ltbo%3D
T Hayashi A Yamamoto E Hagihara Y Ito (1986) Tetrahedron Lett 27 191 Occurrence Handle10.1016/S0040-4039(00)83974-X Occurrence Handle1:CAS:528:DyaL28XmtVyit7k%3D
T Hayashi K Kanechira T Hagihara M Kumada (1988) J Org Chem 53 113 Occurrence Handle10.1021/jo00236a023 Occurrence Handle1:CAS:528:DyaL1cXjvVSkuw%3D%3D
S Toma B Gotov I Kmentova E Solcaniova (2000) Green Chem 2 149 Occurrence Handle10.1039/b002124p Occurrence Handle1:CAS:528:DC%2BD3cXltVOlu70%3D
I Kmentova B Gotov E Solcaniova S Toma (2002) Green Chem 4 103 Occurrence Handle10.1039/b109178f Occurrence Handle1:CAS:528:DC%2BD38XivVaktro%3D
F-T Luo E Negishi (1985) Tetrahedron Lett 26 2177 Occurrence Handle10.1016/S0040-4039(00)98955-X Occurrence Handle1:CAS:528:DyaL2MXlsVWks7g%3D
M Braun F Laicher T Meier (2000) Angew Chem Int Ed 39 3494 Occurrence Handle10.1002/1521-3773(20001002)39:19<3494::AID-ANIE3494>3.0.CO;2-4 Occurrence Handle1:CAS:528:DC%2BD3MXpvVegug%3D%3D
Tsuji J, Minami I, Shimizu I (1983) Chem Lett 1325
K Hiroi J Abe K Suya S Sato T Komayama (1994) J Org Chem 59 203 Occurrence Handle10.1021/jo00080a033 Occurrence Handle1:CAS:528:DyaK2cXhvFSmtb0%3D
KE Atkins WE Walker RM Manyik (1970) Tetrahedron Lett 11 3821 Occurrence Handle10.1016/S0040-4039(01)98599-5
Bergbreiter DE, Weatherford DA (1989) J Chem Soc Chem Commun, 883
JP Haudegong Y Chauvin DJ Commereuc (1979) J Org Chem 44 3063 Occurrence Handle10.1021/jo01331a020
M Kimura R Mujai N Tamigawa S Tanaka Y Tamaru (2003) Tetrahedron 59 7767 Occurrence Handle10.1016/S0040-4020(03)01234-1 Occurrence Handle1:CAS:528:DC%2BD3sXnt12jtL0%3D
M Kimura Y Horino R Mukai S Tanaka Y Tamura (2001) J Am Chem Soc 123 10401 Occurrence Handle10.1021/ja011656a Occurrence Handle1:CAS:528:DC%2BD3MXntVygt7w%3D
S Chandrasekhar V Jagadeswar B Sarith C Narsihmulu (2005) J Org Chem 70 6506 Occurrence Handle10.1021/jo0505728 Occurrence Handle1:CAS:528:DC%2BD2MXls1Ogurg%3D
Me\(\breve {\rm c}\)iarová M, Toma \(\breve {\rm S}\) (2006) Chem Eur J 13: 1268
Kulkarni MG, Davawala S, Anirudha K, Dhanjay S (2004) 2919
CF Bender RA Widenhoefer (2005) J Am Chem Soc 127 1070 Occurrence Handle10.1021/ja043278q Occurrence Handle1:CAS:528:DC%2BD2MXitlGmtw%3D%3D
D Necas M Tursky M Kotora (2004) J Am Chem Soc 126 10222 Occurrence Handle10.1021/ja047320t Occurrence Handle1:CAS:528:DC%2BD2cXmtFOltLo%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hut’ka, M., Toma, Š. Pd-Catalyzed α-Allylation of 2-Phenylpropanal and other Carbonyl Compounds with Allyl Alcohol and Allyl Acetates/Carbonates in Ionic Liquids. Monatsh. Chem. 138, 1175–1179 (2007). https://doi.org/10.1007/s00706-007-0710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-007-0710-6