Abstract
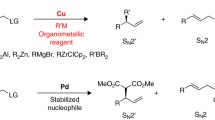
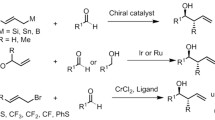
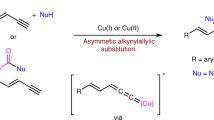
This chapter summarises recent progress in Cu-catalysed asymmetric allylic alkylation (AAA) with organometallic compounds, including Grignard, organolithium, organoaluminium, organozinc and organozirconium reagents. New reaction conditions and chiral ligands that improve these transformations or allow to overcome previous limitations associated with chemo-, regio- and enantioselectivities will be described. Moreover, a description of new ligands and conditions for the introduction of previously elusive nucleophiles, such as highly reactive organolithium compounds, is included together with a brief mechanistic discussion. Additionally, new challenging substrates which provide densely functionalised building blocks, as well as new synthetic applications that take advantage of the terminal olefin formed in these reactions, will be described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Blaser HU, Federsel HJ (2010) Asymmetric catalysis on industrial scale. Wiley, Weinheim
Ojima I (2000) Catalytic asymmetric synthesis, 2nd edn. Wiley, New York
Jacobsen EN, Pfaltz A, Yamamoto H (1999) Comprehensive asymmetric catalysis I–III. Springer, Berlin
Ojima I (2013) Catalytic asymmetric synthesis. Wiley, Weinheim
Karlström ASE, Bäckvall JE (2002) In: Krause N (ed) Copper-Mediated Enantioselective Substitution Reactions. Modern organocopper chemistry. Wiley, Weinheim, pp 259–288
Yorimitsu H, Oshima K (2005) Recent progress in asymmetric allylic substitutions catalyzed by chiral copper complexes. Angew Chem Int Ed 44:4435–4439
Geurts K, Fletcher SP, van Zijl AW, Minnaard AJ, Feringa BL (2008) Copper catalyzed asymmetric allylic substitution reactions with organozinc and Grignard reagents. Pure Appl Chem 80:1025–1037
Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL (2008) Catalytic asymmetric conjugate addition and allylic alkylation with Grignard reagent. Chem Rev 108:2824–2852
Alexakis A, Bäckvall JE, Krause N, Pamies O, Dieguez M (2008) Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions. Chem Rev 108:2796–2823
Falciola CA, Alexakis A (2008) Copper catalyzed asymmetric allylic alkylation. Eur J Org Chem 22:3765–3780
Langlois JB, Alexakis A (2012) Copper-catalyzed enantioselective allylic substitution. Top Organomet Chem 38:235–268
Baslé O, Denicourt-Nowicki A, Crévisy C, Mauduit M (2014) In: Alexakis A, Krause N, Woodward S (eds) Copper-catalyzed asymmetric synthesis. Wiley, Weinheim
Trost BM, Crawey ML (2003) Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem Rev 103:2921–2944
Trost BM (2004) Asymmetric allylic alkylation, an enabling methodology. J Org Chem 69:5813–5837
Lu Z, Ma SM (2008) Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew Chem Int Ed 47:258–297
Milhau L, Guiry PJ (2012) Palladium-catalyzed enantioselective allylic substitution. Top Organomet Chem 38:95–154
Yoshikai N, Nakamura E (2011) Mechanisms of nucleophilic organocopper(I) reactions. Chem Rev 112:2339–2372
Vanklaveren M, Persson ESM, Delvillar A, Grove DM, Bäckvall JE, van Koten G (1995) Chiral arenethiolatocopper(I) catalyzed substitution reactions of acyclic allylic substrates with Grignard reagents. Tetrahedron Lett 36:3059–3062
Meuzelaar GJ, Karlstrom ASE, van Klaveren M, Persson ESM, del Villar A, van Koten G (2000) Asymmetric Induction in the arenethiolatocopper(I)-catalyzed substitution reaction of Grignard reagents with allylic substrates. Tetrahedron 56:2895–2903
Karlstrom ASE, Huerta FF, Meuzelaar GJ, Bäckvall JE (2001) Ferrocenyl thiolates as ligands in the enantioselective copper-catalyzed substitution of allylic acetates with Grignard reagents. Synlett 923–926
Cotton HK, Norinder J, Bäckvall JE (2006) Screening of ligands in the asymmetric metallocenethiolatocopper(I)-catalyzed allylic substitution with Grignard reagents. Tetrahedron 62:5632–5640
Dübner F, Knockel P (1999) Copper(I)-catalyzed enantioselective substitution of allyl chlorides with diorganozinc compounds. Angew Chem Int Ed 38:379–381
Malda H, van Zijl AW, Arnold LA, Feringa BL (2001) Enantioselective copper-catalyzed allylic alkylation with dialkylzincs using phosphoramidite ligands. Org Lett 3:1169–1171
Feringa BL, Badorrey R, Pena D, Harutyunyan SR, Minnaard AJ (2004) Copper-catalyzed asymmetric conjugate addition of Grignard reagents to cyclic enones. Proc Natl Acad Sci U S A 101:5834–5838
Alexakis A, Malan C, Lea L, Benhaim C, Fournioux X (2001) Enantioselective copper catalyzed SN2′ substitution with Grignard reagents. Synlett 927–930
Lölsberg W, Ye S, Schmalz HG (2010) Enantioselective copper catalyzed allylic alkylation of cinnamyl chlorides by Grignard reagent using chiral phosphine-phosphite ligands. Adv Synth Catal 352:2023–2031
Lölsberg W, Werle S, Neudörfl JM, Schmalz HG (2012) An enantioselective total synthesis of helioporins C and E. Org Lett 14:5996–5999
Fang F, Zhang H, Xie F, Yang G, Zhang W (2010) Highly enantioselective copper-catalyzed allylic alkylation with atropos phosphoramidites bearing a D2-symmetric biphenyl backbone. Tetrahedron 66:3593–3598
Magre M, Mazuela J, Diéguez M, Pàmies O, Alexakis A (2012) Furanoside phosphite-phosphoroamidite and diphosphoroamidite ligands applied to asymmetric Cu-catalyzed allylic substitution reactions. Tetrahedron Asymmetry 23:67–71
Magrez M, Le Guen Y, Baslé O, Crévisy C, Mauduit M (2013) Bidentate hydroxyalkyl NHC ligands for the copper-catalyzed asymmetric allylic substitution of allyl phosphates with Grignard reagents. Chem Eur J 19:1199–1203
Van der Molen NC, Tiemersma-Wegman TD, Fañanás-Mastral M, Feringa BL (2015) Regio- and enantioselective copper-catalyzed allylic alkylation of ortho-substituted cinnamyl bromides with Grignard reagents. J Org Chem 80:4981–4984
Hornillos V, Pérez M, Fañanás-Mastral M, Feringa BL (2013) Copper-catalyzed enantioselective allyl-allyl cross-coupling. J Am Chem Soc 135:2140–2143
Selim KB, Matsumoto Y, Yamada K, Tomioka K (2009) Efficient chiral N-heterocyclic carbene/copper(I)-catalyzed asymmetric allylic arylation with aryl Grignard reagents. Angew Chem Int Ed 48:8733–8735
Selim KB, Nakanishi H, Matsumoto Y, Yamamoto Y, Yamada K, Tomioka K (2011) Chiral N-heterocyclic carbene-copper(I)-catalyzed asymmetric allylic arylation of aliphatic allylic bromides: steric and electronic effects on γ-selectivity. J Org Chem 76:1398–1408
Huang Y, Fañanás-Mastral M, Minnaard AJ, Feringa BL (2013) A novel catalytic asymmetric route towards skipped dienes with a methyl-substituted central stereogenic carbon. Chem Commun 49:3309–3311
Li H, Alexakis A (2012) Enyne chlorides: Substrates for copper catalyzed asymmetric allylic alkylation. Angew Chem Int Ed 51:1055–1058
Dabrowski JA, Gao F, Hoveyda AH (2011) Enantioselective synthesis of alkyne-substituted quaternary carbon stereogenic centers through NHC−Cu-catalyzed allylic substitution reactions with (iBu)2(alkynyl)aluminum reagents. J Am Chem Soc 133:4778–4781
Fananas-Mastral M, Feringa BL (2010) Copper-catalyzed regio- and enantioselective synthesis of chiral enol acetates and beta-substituted aldehydes. J Am Chem Soc 132:13152–13153
Giannerini M, Fañanás-Mastral M, Feringa BL (2012) Z-selective copper-catalyzed asymmetric allylic alkylation with Grignard reagents. J Am Chem Soc 134:4108–4111
Li H, Müller D, Guénée L, Alexakis A (2012) Copper-catalyzed enantioselective synthesis of axially chiral allenes. Org Lett 14:5880–5883
Den Hartog T, Macía B, Minnaard AJ, Feringa BL (2010) Copper catalyzed asymmetric allylic alkylation of halocrotonates: efficient synthesis of versatile chiral multifunctional building blocks. Adv Synth Catal 352:999–1013
Hornillos V, Pérez M, Fañanás-Mastral M, Feringa BL (2013) Cu-catalyzed asymmetric allylic alkylation of phosphonates and phosphine oxides with Grignard reagents. Chem Eur J 19:5432–5441
Hornillos V, van Zijl AW, Feringa BL (2012) Catalytic asymmetric synthesis of chromenes and tetrahydroquinolines via sequential allylic alkylation and intramolecular Heck coupling. Chem Commun 48:3712–3714
Teichert JF, Zhang S, van Zijl AW, Slaa JW, Minnaard AJ, Feringa BL (2010) Asymmetric allylic alkylation in combination with ring closing metathesis for the preparation of chiral N-heterocycles. Org Lett 12:4658–4660
Mao B, Geurts K, Fañanás-Mastral M, van Zijl AW, Fletcher SP, Minaard AJ, Feringa BL (2011) Catalytic enantioselective synthesis of naturally occurring butenolides via hetero-allylic alkylation and ring closing metathesis. Org Lett 13:948–951
Geurts K, Fletcher SP, Feringa BL (2006) Copper catalyzed asymmetric synthesis of chiral allylic esters. J Am Chem Soc 128:15572–15573
Mao B, Fañanás-Mastral M, Lutz M, Feringa BL (2013) Diversity-oriented enantioselective synthesis of highly functionalized cyclic and bicyclic alcohols. Chem Eur J 19:761–770
Giacomina F, Riat D, Alexakis A (2010) o-Ethylenic allylic substrates as alternatives to cyclic substrates in copper- and iridium-catalyzed asymmetric allylic alkylation. Org Lett 12:1156–1159
Giacomina F, Alexakis A (2013) Construction of enantioenriched cyclic compounds by asymmetric allylic alkylation and ring-closing metathesis. Eur J Org Chem 29:6710–6721
Fañanás-Mastral M, ter Horst B, Minnaard AJ, Feringa BL (2011) Stereoselective synthesis of syn and anti 1,2-hydroxyalkyl moieties by Cu-catalyzed asymmetric allylic alkylation. Chem Commun 47:5843–5845
Pérez M, Fañanas-Mastral M, Bos PH, Rudolph A, Harutyunyan SR, Feringa BL (2011) Catalytic asymmetric carbon–carbon bond formation via allylic alkylations with organolithium compounds. Nat Chem 3:377–381
Hodgson DM (2003) Organolithiums in enantioselective synthesis. Springer, Germany
Giannerini M, Fañanás-Mastral M, Feringa BL (2013) Direct catalytic cross-coupling of organolithium compounds. Nat Chem 5:667–672
Hornillos V, Giannerini M, Vila C, Fañanás-Mastral M, Feringa BL (2015) Direct catalytic cross-coupling of alkenyllithium compounds. Chem Sci 6:1394–1398
Firth JD, O'Brien P (2015) Cross-coupling knows no limits: assessing the synthetic potential of the palladium-catalysed cross-coupling of organolithiums. ChemCatChem 7:395–397
Kiyotsuka Y, Kobayashi Y (2010) Highly efficient substitution of allylic picolinates with copper reagents derived from aryl-, alkenyl-, furyl-, and thienyl-lithiums. Tetrahedron 66:676–684
Fañanás-Mastral M, Pérez M, Bos PH, Rudolph A, Harutyunyan SR, Feringa BL (2012) Enantioselective synthesis of tertiary and quaternary stereogenic centers: copper/phosphoramidite-catalyzed allylic alkylation with organolithium reagents. Angew Chem Int Ed 51:1922–1925
Teichert JF, Feringa BL (2010) Phosphoramidites: privileged ligands in asymmetric catalysis. Angew Chem Int Ed 49:2486–2528
Guduguntla S, Fañanás-Mastral M, Feringa BL (2013) Synthesis of optically active β- or γ-alkyl-substituted alcohols through copper-catalyzed asymmetric allylic alkylation with organolithium reagents. J Org Chem 78:8274–8280
Hawner C, Alexakis A (2010) Metal-catalyzed asymmetric conjugate addition reaction: formation of quaternary stereocenter. Chem Commun 46:7295–7306
Trost BM, Jiang C (2006) Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis 369–396
Mei TS, Patel HH, Sigman MS (2014) Enantioselective construction of remote quaternary stereocentres. Nature 508:340–344
Prakash J, Marek I (2011) Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem Commun 47:4593–4623
Fañanás-Mastral M, Vitale R, Pérez M, Feringa BL (2015) Enantioselective synthesis of all-carbon quaternary stereogenic centers via copper-catalyzed asymmetric allylic alkylation of (Z)-allyl bromides with organolithium reagents. Chem Eur J 21:4209–4212
Gao F, McGrath KP, Lee Y, Hoveyda AH (2010) Synthesis of quaternary carbon stereogenic centers through enantioselective Cu-catalyzed allylic substitutions with vinylaluminum reagents. J Am Chem Soc 132:14315–14320
Hojoh K, Shido Y, Ohmiya H, Sawamura M (2014) Construction of quaternary stereogenic carbon centers through copper-catalyzed enantioselective allylic cross-coupling with alkylboranes. Angew Chem Int Ed 53:4954–4958
Tseng CC, Paisley SD, Goering HL (1986) Alkylation of allylic derivatives. 11. Copper(I)-catalyzed cross coupling of allylic carboxylates with Grignard reagents. J Org Chem 51:2884–2891
Von dem Bussche-Hünnefeld JL, Seebach D (1992) Enantioselective preparation of sec. Alcohols from aldehydes and dialkyl zinc compounds, generated in situ from Grignard reagents, using substoichiometric amounts of TADDOL-titanates. Tetrahedron 48:5719–5730
Pérez M, Fañanás-Mastral M, Hornillos V, Rudolph A, Bos PH, Harutyunyan SR, Feringa BL (2012) Asymmetric allylic alkylation of acyclic allylic ethers with organolithium reagents. Chem Eur J 18:11880–11883
Zhang L, Le CM, Lautens M (2014) The Use of silyl ketene acetals and enol ethers in the catalytic enantioselective alkylative ring opening of oxa/aza bicyclic alkenes. Angew Chem Int Ed 53:5951–5954
Fagnou K, Lautens M (2003) Rhodium-catalyzed carbon−carbon bond forming reactions of organometallic compounds. Chem Rev 103:169–196
Yang D, Liang N (2014) Platinum-catalyzed anti-stereocontrolled ring-opening of oxabicyclic alkenes with Grignard reagents. Org Biomol Chem 12:2080–2086
Lautens M, Renaud JL, Hiebert S (2000) Palladium-catalyzed enantioselective alkylative ring opening. J Am Chem Soc 122:1804–1805
Bertozzi F, Pineschi M, Macchia F, Arnold LA, Minnaard AJ, Feringa BL (2002) Copper phosphoramidite catalyzed enantioselective ring-opening of oxabicyclic alkenes: remarkable reversal of stereocontrol. Org Lett 4:2703–2705
Gómez Arrayás R, Cabrera S, Carretero JC (2003) Copper-catalyzed anti-stereocontrolled ring opening of oxabicyclic alkenes with grignard reagents. Org Lett 5:1333–1336
Bos PH, Rudolph A, Perez M, Fañanas-Mastral M, Harutyunyan SR, Feringa BL (2012) Copper-catalyzed asymmetric ring opening of oxabicyclic alkenes with organolithium reagents. Chem Commun 48:1748–1750
Palais L, Bournaud C, Micouin L, Alexakis A (2010) Copper-catalysed ring opening of polycyclic meso-hydrazines with trialkylaluminium reagents and SimplePhos ligands. Chem Eur J 16:2567–2573
Akiyama K, Gao F, Hoveyda AH (2010) Stereoisomerically pure trisubstituted vinylaluminum reagents and their utility in copper-catalyzed enantioselective synthesis of 1,4-dienes containing Z or E alkenes. Angew Chem Int Ed 49:419–423
Gao F, Lee Y, Mandai K, Hoveyda AH (2010) Quaternary carbon stereogenic centers through copper-catalyzed enantioselective allylic substitutions with readily accessible aryl- or heteroaryllithium reagents and aluminum chlorides. Angew Chem Int Ed 49:8370–8374
Dabrowski JA, Haeffner F, Hoveyda AH (2013) Combining NHC–Cu and Brønsted base catalysis: enantioselective allylic substitution/conjugate additions with alkynylaluminum reagents and stereospecific isomerization of the products to trisubstituted allenes. Angew Chem Int Ed 52:7694–7699
Harada A, Yusuke M, Tatsunori S, Ohmiya H, Sawamura M (2014) Copper-catalyzed enantioselective allylic alkylation of terminal alkyne pronucleophiles. J Am Chem Soc 136:13932–13939
Jennequin T, Wencel-Delord J, Rix D, Daubignard J, Crévisy C, Mauduit M (2010) Chelating hydroxyalkyl NHC as efficient chiral ligands for room-temperature copper-catalyzed asymmetric allylic alkylation. Synlett 11:1661–1665
Jahier-Diallo C, Morin MST, Queval P, Rouen M, Artur I, Querard P, Toupet L, Crévisy C, Baslé O, Mauduit M (2015) Multicomponent synthesis of chiral bidentate unsymmetrical unsaturated N-heterocyclic carbenes: copper-catalyzed asymmetric C–C Bond formation. Chem Eur J 21:993–997
Pellissier H (2011) Organocatalyzed dynamic kinetic resolution. Adv Synth Catal 353:659–676
Nakano K, Kitamura M (2014) Dynamic kinetic resolution (DKR). Wiley, Weinhelm
Huerta FF, Minidisa ABE, Bäckvall JE (2001) Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem Soc Rev 30:321–331
Ito H, Kunii S, Sawamura M (2010) Direct enantio-convergent transformation of racemic substrates without racemization or symmetrisation. Nat Chem 2:972–976
Cherney AH, Kadunce NT, Reisman SE (2015) Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem Rev 115:9587–9652
Ladjel C, Fuchs N, Gremaud L, Alexakis A (2010) Copper-catalysed kinetic resolution of cyclic alkenyl cyclopropane malonates with organoaluminium and grignard reagents. Synlett 02:0317–0320
Langlois JB, Alexakis A (2010) Copper-catalyzed asymmetric allylic alkylation of racemic cyclic substrates: application of dynamic kinetic asymmetric transformation (DYKAT). Adv Synth Catal 352:447–457
Langlois JB, Alexakis A (2011) Identification of a valuable kinetic process in copper-catalyzed asymmetric allylic alkylation. Angew Chem Int Ed 50:1877–1881
Langlois JB, Emery D, Mareda J, Alexakis A (2012) Mechanistic identification and improvement of a direct enantioconvergent transformation in copper-catalyzed asymmetric allylic alkylation. Chem Sci 3:1062–1069
Bertozzi F, Crotti P, Macchia F, Pineschi M, Feringa BL (2001) Highly enantioselective regiodivergent and catalytic parallel kinetic resolution. Angew Chem Int Ed 40:930–932
Crotti P, Di Bussolo V, Pineschi M (2011) Copper-catalyzed divergent kinetic resolution of racemic allylic substrates. Chirality 23:703–710
You H, Rideau E, Sidera M, Fletcher SP (2015) Non-stabilized nucleophiles in Cu-catalysed dynamic kinetic asymmetric allylic alkylation. Nature 517:351–355
Sidera M, Fletcher SP (2015) Cu-catalyzed asymmetric addition of sp2-hybridized zirconium nucleophiles to racemic allyl bromides. Chem Commun 51:5044–5047
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hornillos, V., Gualtierotti, JB., Feringa, B.L. (2016). Asymmetric Allylic Substitutions Using Organometallic Reagents. In: Harutyunyan, S. (eds) Progress in Enantioselective Cu(I)-catalyzed Formation of Stereogenic Centers. Topics in Organometallic Chemistry, vol 58. Springer, Cham. https://doi.org/10.1007/3418_2015_165
Download citation
DOI: https://doi.org/10.1007/3418_2015_165
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33412-7
Online ISBN: 978-3-319-33414-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)