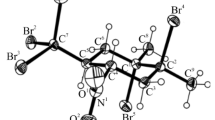
Summary.
Treatment of a number of 2-substituted 1,1,2-tribromocyclopropanes with MeLi at −78°C gave the corresponding 1-bromocyclopropenes, which were reacted with three cyclic dienes to yield the [4 + 2]-cycloadducts. Cycloaddition with 1,3-diphenylisobenzofuran (DPIBF) gave the exo adducts, in most cases in excellent yield, whereas cyclopentadiene afforded endo adducts only, but in moderate yield. In most reactions with furan no adduct was formed, but two 1-bromocyclopropenes derivatives with an aromatic side chain were exceptions and furnished mixtures of exo and endo adducts in moderate yields.
Similar content being viewed by others
References
P Binger P Wedemann UH Brinker (1999) Org Synth 77 254
MS Baird (1988) Top Curr Chem 144 137 Occurrence Handle1:CAS:528:DyaL1MXnsVymsg%3D%3D
MS Baird (1991) Adv Strain Org Chem 1 65 Occurrence Handle1:CAS:528:DyaK38XmtFKr
GL Closs (1966) Adv Alicyclic Chem 1 53 Occurrence Handle1:CAS:528:DyaF2sXktlagtr8%3D
P Binger P Wedemann R Goddard UH Brinker (1996) J Org Chem 61 6462 Occurrence Handle10.1021/jo960728r Occurrence Handle1:CAS:528:DyaK28XltVWksLk%3D
Baird MS, Fitton HL, Clegg W, McCamley A (1993) J Chem Soc, Perkin Trans 1: 321
PJ Garratt A Tsotinis (1990) J Org Chem 55 84 Occurrence Handle1:CAS:528:DyaK3cXjsF2mtQ%3D%3D
Banwell MG, Berak M, Hockless DCR (1996) J Chem Soc, Perkin Trans 1: 2217
GL Closs KD Krantz (1966) J Org Chem 31 638 Occurrence Handle1:CAS:528:DyaF28XosVWrsA%3D%3D
WA Rendall M Torres OP Strausz (1985) J Org Chem 50 3034 Occurrence Handle10.1021/jo00217a002 Occurrence Handle1:CAS:528:DyaL2MXkvVOht74%3D
Reinhoudt DN, Smael P, Van Tilborg WJM, Visser JP (1973) Tetrahedron Lett 3755
JE Baldwin VP Reddy (1989) J Org Chem 54 5264 Occurrence Handle10.1021/jo00283a018 Occurrence Handle1:CAS:528:DyaL1MXmtFeqtbk%3D
Y Apeloig D Arad M Kapon M Wallerstein (1987) Tetrahedron Lett 28 5917 Occurrence Handle10.1016/S0040-4039(01)81090-X Occurrence Handle1:CAS:528:DyaL1cXltV2ru74%3D
WE Billups EW Casserly BE Arney SuffixJr (1984) J Am Chem Soc 106 440 Occurrence Handle1:CAS:528:DyaL2cXltFaisw%3D%3D
WE Billups BE Arney SuffixJr LJ Lin (1984) J Org Chem 49 3436 Occurrence Handle10.1021/jo00192a055 Occurrence Handle1:CAS:528:DyaL2cXltFSnu78%3D
P Müller C Nguyen Thi Huong (1980) Tetrahedron Lett 21 2145
W Ng D Wege (1996) Tetrahedron Lett 37 6797 Occurrence Handle1:CAS:528:DyaK28XlslGjsLg%3D
AR Al Dulayymi MS Baird (1996) Tetrahedron 52 10955 Occurrence Handle1:CAS:528:DyaK28XltVGhtLw%3D
G-A Lee J Chen C-S Chen C-S Shiau C-H Cherng (1996) J Chin Chem Soc (Taipei) 43 297 Occurrence Handle1:CAS:528:DyaK28XjvFyqsbc%3D
B Halton MD Diggins AJ Kay (1992) J Org Chem 57 4080 Occurrence Handle10.1021/jo00041a008 Occurrence Handle1:CAS:528:DyaK38XlslWis7w%3D
LK Sydnes E Bakstad (1997) Acta Chem Scand 51 1132 Occurrence Handle1:CAS:528:DyaK2sXntV2hu7o%3D
E Bakstad AS Olsen M Sandberg LK Sydnes (1999) Acta Chem Scand 53 465 Occurrence Handle1:CAS:528:DyaK1MXkvVKltbc%3D
LK Sydnes E Bakstad (1996) Acta Chem Scand 50 446
LK Sydnes KFS Alnes N Erdogan (2005) Chem Monthly 136 1737 Occurrence Handle1:CAS:528:DC%2BD2MXhtVGntrjL
BJ Wakefield (1974) The chemistry of organolithium compounds Pergamon Oxford 51
D Seyferth RL Lambert SuffixJr M Massol (1975) J Organomet Chem 88 255 Occurrence Handle1:CAS:528:DyaE2MXksVens78%3D
Zohar E, Ram M, Marek I (2004) Synlett 1288
WE Billups MM Haley GA Lee (1989) Chem Rev 89 1147 Occurrence Handle10.1021/cr00095a011 Occurrence Handle1:CAS:528:DyaL1MXkvFKlur4%3D
MS Baird (2003) Chem Rev 103 1271 Occurrence Handle10.1021/cr010021r Occurrence Handle1:CAS:528:DC%2BD3sXitFGhsLY%3D
Deem ML (1972) Synthesis 675
DA Hrovat WT Borden (1988) J Am Chem Soc 110 7229 Occurrence Handle1:CAS:528:DyaL1cXls12qsrY%3D
KB Wiberg WJ Bartley (1960) J Am Chem Soc 82 6375 Occurrence Handle10.1021/ja01509a045 Occurrence Handle1:CAS:528:DyaF3MXjslOqsQ%3D%3D
W Spiller H Kliesch D Wöhrle S Hackbarth B Röder G Schnurpfeil (1998) J Porphyrins Phthalocyanines 2 145 Occurrence Handle10.1002/(SICI)1099-1409(199803/04)2:2<145::AID-JPP60>3.0.CO;2-2 Occurrence Handle1:CAS:528:DyaK1cXktFelt7g%3D
LM Jackman S Sternhell (1972) Applications of nuclear magnetic resonance spectroscopy in organic chemistry, ed 2 Pergamon Press Oxford 80
K Alder R Hartmann W Roth (1958) Justus Liebigs Ann Chem 613 6 Occurrence Handle1:CAS:528:DyaG1cXhtVeqsbY%3D
JG Martin RK Hill (1961) Chem Rev 61 537 Occurrence Handle10.1021/cr60214a001 Occurrence Handle1:CAS:528:DyaF38XjvVWgug%3D%3D
P Müller G Bernardinelli J Pfyffer D Rodriguez JP Schaller (1988) Helv Chim Acta 71 544
K Alder G Stein (1937) Angew Chem 50 510 Occurrence Handle1:CAS:528:DyaA2sXltVOktw%3D%3D
GL Closs LE Closs WA Böll (1963) J Am Chem Soc 85 3796 Occurrence Handle1:CAS:528:DyaF2cXptlyr
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1989) Vogel’s Textbook of Practical Organic Chemistry, ed 5, Longman, Singapore, pp 444–445
Braun S, Kalinowski H-O, Berger S (1998) 150 and more basic NMR experiments: a practical course, ed 2, Wiley-VCH, Weinheim
MS Baird W Nethercott (1983) Tetrahedron Lett 24 605 Occurrence Handle10.1016/S0040-4039(00)81476-8 Occurrence Handle1:CAS:528:DyaL3sXlslGhsbs%3D
Baird MS, Hussain HH, Nethercott W (1986) J Chem Soc, Perkin Trans 1: 1845
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alnes, K., Sydnes, L. Synthesis and Trapping of Some Substituted 1-Bromocyclopropenes. Monatsh. Chem. 137, 483–500 (2006). https://doi.org/10.1007/s00706-006-0441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-006-0441-0