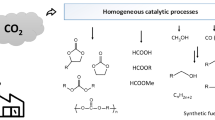
Summary.
Organic carbamates classically have been synthesized using harmful and toxic reagents like phosgene, its derivatives, and carbon monoxide. Recently, carbon dioxide was used as a cheap and harmless reagent for the synthesis of organic carbamates in the gaseous or supercritical state, or in an electrochemical process, or organic carbonates as sources of carbon dioxide as an alternative to the harmful reagents. The present review will deal with the extensive use of carbon dioxide in the synthesis of organic carbamates.
Similar content being viewed by others
References
P Adams FA Baron (1965) Chem Rev 65 567 Occurrence Handle10.1021/cr60237a002 Occurrence Handle1:CAS:528:DyaF2MXksl2qsLw%3D
a) Ray S, Chaturvedi D (2004) Drugs of the Future 29: 343; b) Ray S, Pathak SR, Chaturvedi D (2005) Drugs of the Future 30: 161
a) Mateen A, Chapalamadugu S, Kashar B, Bathi AR, Chaudhary GR (1994) Biol Degrad Biorem Toxic Chem 198; b) Wigfield YY (1996) Food Sci Technol (NY) 77: 1501
a) Snieckus V (1990) Chem Rev 90: 879; b) Cleraland WW, Andrews TS, Gutteridge S, Hartman FC, Lorimer GH (1998) Chem Rev 98: 549; c) Tanaka F (2002) Chem Rev 102: 4885
Greene TW, Wuts PGM (1999) Protecting Groups in Organic Synthesis 3rd edn. John Wiley and Sons, New York, 503
a) Mayer JP, Lewis GS, Curtius MJ, Zhang J (1997) Tetrahedron Lett 38: 8445; b) Buchstaller HP (1998) Tetrahedron 54: 3465; c) Holte PT, Thijs L, Zwanenburg B (1998) Tetrahedron Lett 39: 7404
a) Matsui T, Kondo T, Nishita Y, Itadani S, Tsuruta H, Fujita S, Omawari N, Sakai M, Nakazawa S, Ogata A, Mori H, Ohno H, Obata T, Nakai H, Toda M (2002) Bioorg Med Chem Lett 12: 907; b) Seko T, Kato M, Kohno H, Ono S, Hashimura K, Takimizu H, Nakai K, Maegawa H, Katsube N, Toda M (2002) Bioorg Med Chem Lett 12: 915
DPN Satchell RS Satchell (1975) Chem Soc Rev 4 231 Occurrence Handle10.1039/cs9750400231 Occurrence Handle1:CAS:528:DyaE2MXkvFGktr0%3D
S Raucher DS Jones (1985) Synth Commun 15 1025 Occurrence Handle1:CAS:528:DyaL28XitVKgsro%3D
a) Leung TW, Domblk BD (1992) Chem Commun 3: 205; b) Mizuno T, Takahashi J, Ogawa A (2002) Tetrahedron 58: 7805
R Mahe Y Sasaki C Bruneau PH Dixneuf (1989) J Org Chem 54 1518 Occurrence Handle10.1021/jo00268a008 Occurrence Handle1:CAS:528:DyaL1MXhsFektrw%3D
MA Yoshida N Hara S Okuyama (2000) Chem Commun 2 151
a) Casedei MA, Inesi A, Moracci FM, Rossi L (1996) Chem Commun 2575; b) Casadei MA, Moracci FM, Zappia G, Inesi A, Rossi L (1997) J Org Chem 62: 6754; c) Feroci M, Inesi A, Rossi L (2000) Tetrahedron Lett 41: 963
H Arakawa M Aresta JN Armor MA Barteau EJ Becman AT Bell JE Bercaw C Creutz E Dinjus DA Dixon K Domen DL Dubois J Eckert E Fijita DH Gibson WA Goddard DW Goodman J Kellr GJ Kubas HH Kung JE Lyons LE Manzer TJ Marks K Morokuma KM Nicholas R Periana L Que JR Nielson WMH Sachtler LD Schimdt A Sen GA Somarjai PC Stair BR Stults W Tumas (2001) Chem Rev 101 953 Occurrence Handle1:CAS:528:DC%2BD3MXisFehsLg%3D Occurrence Handle10.1021/cr000018s
Yoshida Y, Inoue S (1977) Chem Lett 1575
a) Yoshida Y, Ishii S, Yamashita T (1984) Chem Lett 1571; b) Yoshida Y, Ishii S, Watanabe M, Yamashita T (1989) Bull Chem Soc Jpn 62: 1534
S Ishii H Nakayama Y Yoshida T Yamashita (1989) Bull Chem Soc Jpn 62 455 Occurrence Handle1:CAS:528:DyaL1MXlsFart7s%3D
Yoshida Y, Inoue S (1978) Chem Lett 139
Y Yoshida S Inoue (1978) Bull Chem Soc Jpn 51 559 Occurrence Handle1:CAS:528:DyaE1cXhsFaju70%3D
Asano T, Saito N, Ito S, Hatakeda K, Toda T (1978) Chem Lett 311
Yoshida Y, Inoue S (1979) J Chem Soc Perkin Trans I 3146
E Christ F Kojima T Aida S Inoue (1986) J Am Chem Soc 108 391
Toda T (1977) Chem Lett 957
N Saito K Hatakeda S Ito T Asano T Toda (1986) Bull Chem Soc Jpn 59 1629 Occurrence Handle1:CAS:528:DyaL2sXhtF2ksbk%3D
M Yoshida M Ohshima T Toda (1993) Heterocycles 35 623 Occurrence Handle1:CAS:528:DyaK2cXitFOnur4%3D
Hori Y, Nagano Y, Nakao J, Fukuhara T, Taniguchi H (1986) Chem Express 224
M Aresta E Quaranta (1992) Tetrahedron 48 1515 Occurrence Handle10.1016/S0040-4020(01)92239-2 Occurrence Handle1:CAS:528:DyaK38XhvFelu74%3D
a) McGhee WD, Pan Y, Riley DP (1994) Chem Commun 699; b) McGhee WD, Riley DP, Christ K, Pan Y, Parnas B (1995) J Org Chem 60: 2820
a) McGhee WD, Riley DP, Christ MKM (1993) Organometallics 12: 1429; b) Aresta M, Quaranta E (1997) CHEMTECH 32; c) Jessop PG, Ikariya T, Noyori R (1995) Chem Rev 95: 259
ER Perez MO Silva VC Costa UPR Filho DW Franco (2002) Tetrahedron Lett 43 4091 Occurrence Handle10.1016/S0040-4039(02)00697-4 Occurrence Handle1:CAS:528:DC%2BD38Xjsl2iu70%3D
Kubota Y, Kodaka M, Tomohiro T, Okuno H (1993) J Chem Soc Perkin Trans I 5
Tominaga KI, Sasaki Y (2002) Synlett 307
Chaturvedi D, Ray S (2005) Monatsh Chem
Chaturvedi D, Ray S (2005) Monatsh Chem
D Chaturvedi A Kumar S Ray (2003) Tetrahedron Lett 44 7637 Occurrence Handle10.1016/j.tetlet.2003.08.018 Occurrence Handle1:CAS:528:DC%2BD3sXnt1yitrc%3D
Alba M, Choi JC, Sakakura T (2001) Chem Com 2238
Sasaki Y, Dixneuf PH (1986) Chem Com 790
R Mahe Y Sasaki C Bruneau PH Dixneuf (1989) J Org Chem 54 1518 Occurrence Handle10.1021/jo00268a008 Occurrence Handle1:CAS:528:DyaL1MXhsFektrw%3D
S Shim J Chul D Ook C Hoon YKT Youn (1990) Bull Korean Chem Soc 11 468
J Hofer H Doncet C Brunean PH Dixneuf (1991) Tetrahedron Lett 32 7409 Occurrence Handle10.1016/0040-4039(91)80119-Q Occurrence Handle1:CAS:528:DyaK38XhtVGhtL0%3D
Butcher KJ (1994) Synlett 825
RN Salvatore VL Flander D Ha KW Jung (2000) Org Lett 2 2797 Occurrence Handle10.1021/ol006212i Occurrence Handle1:CAS:528:DC%2BD3cXltlans7k%3D
a) Salvatore RN, Shin SI, Nagle AS, Jung KW (2001) J Org Chem 66: 1035; b) Salvatore RN, Chu F, Nagle AS, Kapxhin EA, Cross RM, Jung KW (2002) Tetrahedron 58: 3329
RN Salvatore JA Ledger KW Jung (2001) Tetrahedron Lett 42 6023 Occurrence Handle1:CAS:528:DC%2BD3MXlvFaru74%3D
M Shi YM Shen (2001) Helv Chim Acta 84 3357 Occurrence Handle10.1002/1522-2675(20011114)84:11<3357::AID-HLCA3357>3.0.CO;2-P Occurrence Handle1:CAS:528:DC%2BD3MXptFKqtLY%3D
D Chaturvedi A Kumar S Ray (2002) Synth Commun 32 2651 Occurrence Handle10.1081/SCC-120006028 Occurrence Handle1:CAS:528:DC%2BD38XmslCrs7w%3D
a) Casadei MA, Moracci FM, Zappia G, Inesi A, Rossi L (1997) J Org Chem 62: 6754; b) Feroci M, Inesi A, Rosii L (2000) Tetrahedron Lett 41: 963; c) Feroci M, Casadei AM, Orsini M, Palombi L, Inesi I (2003) J Org Chem 68: 1548
M Feroci A Gennaro A Inesi M Orsini L Palombi (2002) Tetrahedron Lett 43 5863 Occurrence Handle10.1016/S0040-4039(02)01186-3 Occurrence Handle1:CAS:528:DC%2BD38Xls1Gktbs%3D
Tascedda P, Dunach E (2000) Chem Commun 449
Yoshida M, Hara N, Okuyama S (2000) Chem Commun 151
Anon, Res, Discl. (1987) 275: 162; Chem Abstr (1988) 108: 167429g; b) Romano U, Fornasari G, Di Gioa Chem Abstr (1982) 97: 144607d; c) Mukai T, Suenobu K, Mitsuru M Japan Kokai 7714,745, Chem Abstr (1977) 87: 52691e
a) Frulla FF, Stuber AF, Witman JP US patent 4,550,188. Chem Abstr (1986) 104: 224725u; b) Guragio AE US 4,268,684, Chem Abstr (1981) 96,97407k; c) Guragio AE US 4.268,683 Chem Abstr (1981) 95: 168832h
F Porta S Cenini M Pizzoti C Crotti (1985) Gazz Chim Ital 115 275 Occurrence Handle1:CAS:528:DyaL2MXls1Sju74%3D
a) Massi Mauri M, Romano U, Rivetti F (1985) Ing Chim Ital 21: 6; b) Tundo P, Selva M (2002) Acc Chem Res 35: 706
a) Aresta M, Quaranta E (1988) J Org Chem 53: 4153; b) Aresta M, Quaranta E Ital Pat 1198206; c) Aresta M, Quaranta E, Ital Pat 22740, A/89; d) Aresta M, Quaranta E (1991) Tetrahedron 47: 9489
a) Wright HB, Moore MB (1948) J Am Chem Soc 70: 3865; b) Wolfe JK, Temple KL (1948) J Am Chem Soc 70: 1414; c) Zahraduich KR (1959) Chem Tech 11: 546; d) Lallu JB, Masson J, Guerin H, Roger MF (1972) Bull Soc Chim Fr 1311; e) Carretti E, Dei L, Baglioni P, Weiss R (2003) J Am Chem Soc 125: 5121
a) Tsuda T, Washita H, Watanabe K, Miwa M, Saegusa T (1978) Chem Commun 815; b) Tsuda T, Watanabe K, Miyata K, Yamamoto H (1981) Inorg Chem 20: 2778; c) Yoshida Y, Ishi S, Yamashita T (1984) Chem Lett 1571
M Aresta C Berloco E Quaranta (1995) Tetrahedron 51 8073 Occurrence Handle10.1016/0040-4020(95)00424-7 Occurrence Handle1:CAS:528:DyaK2MXntFansLc%3D
Ghosh AK, Mckee SP, Doung TT, Thompson WJ (1992) Chem Commun 1308
AK Ghosh TT Doung SP McKee WJ Thomson (1992) Tetrahedron Lett 33 2781 Occurrence Handle1:CAS:528:DyaK38XkvFSlsrY%3D
Chandrasekher S, Chandrauch L, Reddy CR, Reddy MV (2000) Chem Lett 780
S Chandrasekhar BN Bubu CR Reddy (2003) Tetrahedron Lett 44 2057 Occurrence Handle1:CAS:528:DC%2BD3sXht1yltrw%3D
YJ Jung YM Chang JH Lee CM Yoon (2002) Tetrahedron Lett 43 8735 Occurrence Handle1:CAS:528:DC%2BD38XosVKitLo%3D
MS Castro P Douningnez JV Sinisterra (2000) Tetrahedron 56 1387
M Pozo V Gotor (1993) Tetrahedron 49 4321 Occurrence Handle1:CAS:528:DyaK3sXmtVaqtLg%3D
I Vauthey F Valot C Gozzi F Fache M Leunaire (2000) Tetrahedron Lett 41 6347 Occurrence Handle10.1016/S0040-4039(00)01051-0 Occurrence Handle1:CAS:528:DC%2BD3cXls1Wnt74%3D
Gupte SP, Shivarkar AB, Chaudhari RV (2001) Chem Commun 2620
M Selva P Tundo A Perosa (2002) Tetrahedron Lett 43 1217 Occurrence Handle10.1016/S0040-4039(01)02390-5 Occurrence Handle1:CAS:528:DC%2BD38XpslGlsQ%3D%3D
M Curini F Epifano F Malterse O Rosate (2002) Tetrahedron Lett 43 4895 Occurrence Handle1:CAS:528:DC%2BD38XkvVegu7k%3D
T Sima S Guo F Shi Y Deng (2002) Tetrahedron Lett 43 8145 Occurrence Handle10.1016/S0040-4039(02)01943-3 Occurrence Handle1:CAS:528:DC%2BD38XnvVymurw%3D
LF Garcia-Alles F Morris V Gotor (1993) Tetrahedron Lett 34 6337 Occurrence Handle1:CAS:528:DyaK2cXhtlGjtrw%3D Occurrence Handle10.1016/S0040-4039(00)73746-4
Iwasaki T, Kihara N, Eudo T (2000) Bull Chem Soc Jpn 73: 713; b) Alsima J, Rabanal F, Chiva C, Giralt E, Albercio F (1998) Tetrahedron 54: 10125
M Pittelkow R Lewinsky JB Christensen (2002) Synthesis 15 2195
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaturvedi, D., Ray, S. Versatile Use of Carbon Dioxide in the Synthesis of Carbamates. Monatsh. Chem. 137, 127–145 (2006). https://doi.org/10.1007/s00706-005-0423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-005-0423-7