Summary.
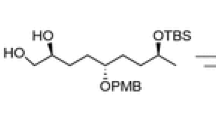
(αR,6R,7R)-7-(1-Acetoxyethyl)-3-methyl-2-isoxacephem-4-carboxylic acid and its enantiomer have been prepared. The ring systems were formed from the corresponding enantiomerically pure N-unsubstituted β-lactams. The reduction of methyl [(αR,2S,3R)-3-(1-acetoxyethyl)-1-(4-methoxyphenyl)-4-oxoazetidine-2-carboxylate] has been solved via a hemi-acetal. The structure and the configuration of a new stereogenic center in this intermediate was predicted by using 2D NMR technique and unambiguously proven by x-ray.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánta, Z., Nagy, J., Párkányi, L. et al. Synthesis of Enantiomerically Pure 2-Isoxacephems. Monatshefte für Chemie 135, 671–684 (2004). https://doi.org/10.1007/s00706-003-0135-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-003-0135-9