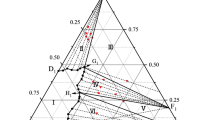
Summary.
The diagram of the ternary system Mg2+/Cl−, SO4 2−–H2O was established at 15°C by means of analytical and conductimetric measurements. Three compounds were found in this diagram, which are MgSO4·6H2O, MgSO4·7H2O, and MgCl2·6H2O. The solubility field of MgSO4·7H2O is important whereas those of MgSO4·6H2O and MgCl2·6H2O are small. The compositions (mass-%) of the two invariant points determined by the two methods are: MgSO4:MgCl2=2.73:33.80 and MgSO4: MgCl2=3.38:28.91. Both the measured and the calculated isotherm at 15°C have been used for modelling of the diagram Mg2+/Cl−, SO4 2−–H2O between 0 and 35°C. The polythermal invariant point was approximately located between 15 and 10°C.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Corresponding author. E-mail: ariguib@planet.tn
Received October 16, 2002; accepted (revised) December 3, 2002 Published online April 24, 2003
RID="a"
ID="a" Dedicated to Prof. Dr. Heinz Gamsjäger on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Bousmina, F., Zayani, L., Ben Hassen-Chehimi, D. et al. Experimental Determination of the Isotherm at 15°C of the System Mg2+/Cl−, SO4 2–H2O. Monatshefte für Chemie 134, 763–768 (2003). https://doi.org/10.1007/s00706-002-0589-1
Issue Date:
DOI: https://doi.org/10.1007/s00706-002-0589-1