Summary.
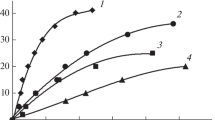
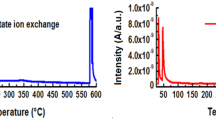
Unpromoted cobalt molybdate was prepared from Co(NO3)2·6H2O and (NH4)6Mo7O24·4H2O, then calcined between 350 and 600°C for 5 h. K2O (10 w%), as a promoter, was added to the calcined sample at 350°C from two different sources (i.e. KOH and KNO3) and was subjected to further calcination at 350°C for 5 h. The catalytic activity of unpromoted catalysts towards the vapour phase decomposition of CH3COOH was greatly influenced by the increase in the calcination temperature. This is attributed to the diminution of both S BET and their dual acidic–basic characters. The promoted sample from the KOH source was found to be the most active of the catalysts studied. This is due to its high population of both acidic–basic surface sites and the formation of two new phases. XRD and FTIR analyses of the used catalysts, after the decomposition reaction of acetic acid, showed a remarkable change in its structure compared with the parent samples.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
E-mail: shalawy99@yahoo.com
Received May 8, 2002; accepted (revised) July 9, 2002
Rights and permissions
About this article
Cite this article
Halawy, S. Unpromoted and K2O-Promoted Cobalt Molybdate as Catalysts for the Decomposition of Acetic Acid. Monatshefte für Chemie 134, 371–380 (2003). https://doi.org/10.1007/s00706-002-0520-9
Issue Date:
DOI: https://doi.org/10.1007/s00706-002-0520-9