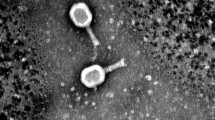
Abstract
Uropathogenic Escherichia coli (UPEC) is the most common causative agent of urinary tract infections, and strains that are resistant to antibiotics are a major problem in treating these infections. Phage therapy is a promising alternative approach that can be used to treat infections caused by polyresistant bacterial strains. In the present study, 16 bacteriophages isolated from sewage and surface water were investigated. Phage host specificity was tested on a collection of 77 UPEC strains. The phages infected 2–44 strains, and 80% of the strains were infected by at least one phage. The susceptible E. coli strains belonged predominantly to the B2 phylogenetic group, including strains of two clones, CC131 and CC73, that have a worldwide distribution. All of the phages belonged to class Caudoviricetes and were identified as members of the families Straboviridae, Autographiviridae, and Drexlerviridae and the genera Kagunavirus, Justusliebigvirus, and Murrayvirus. A phage cocktail composed of six phages – four members of the family Straboviridae and two members of the family Autographiviridae – was prepared, and its antibacterial activity was tested in liquid medium. Complete suppression of bacterial growth was observed after 5–22 hours of cultivation, followed by partial regrowth. At 24 hours postinfection, the cocktail suppressed bacterial growth to 43–92% of control values. Similar results were obtained when testing the activity of the phage cocktail in LB and in artificial urine medium. The results indicate that our phage cocktail has potential to inhibit bacterial growth during infection, and they will therefore be preserved in the national phage bank, serving as valuable resources for therapeutic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli, a member of the family Enterobacteriaceae, is the most common causative agent of urinary tract infections (UTIs) worldwide [1, 2]. Despite commensal colonization of the lower intestine of warm-blooded animals, its presence in the urinary tract can lead to acute UTIs and even to recurrent or chronic infections [3]. The uropathogenic E. coli (UPEC) are a broad group of strains with heterologous genomes that carry a diverse set of virulence genes that help these strains to invade the urothelium [1, 4]. The majority of UPEC isolates belong to phylogenetic group B2 and, to a lesser extent, to the D, A, and B1 phylogroups [5,6,7]. In particular, one lineage of phylogroup B2 with the sequence type (ST) ST131 is emerging and becoming widespread globally [8, 9]. The success of ST131 possibly may lie in the multidrug resistance of many of its representatives [10]. An expansion of antibiotic resistance has been also reported among other UPEC strains [3, 11]. Therefore, the need for alternatives to antibiotics for treatment of UTIs is increasing. Phage therapy is one of the strategies that can be used to treat infections caused by polyresistant bacterial strains [12]. Lytic bacteriophages attack bacteria in a strain-specific manner, independently of their antibiotic resistance, and they do not have any of the side effects that are usually associated with antibiotic treatment, e.g., disturbance of the natural microbiota [13]. Phage therapy may also be useful for patients with chronic or recurrent urinary tract infections, which are most common in females. Although these infections are frequently caused by bacterial agents that are susceptible to antibiotics, phage therapy may spare the patients repeated courses of antimicrobial therapy and their inevitable side effects. During therapy, bacteriophages can be used alone or combined with antibiotics, which are usually applied systemically, while the phages are applied locally, directly to the infectious focus [14].
A major advantage of phage therapy is the ease with which therapeutic phages can be isolated from the environment. However, it is crucial to select phages that have a suitable host range, capable of effectively targeting and killing a specific pathogen of interest. Only strictly lytic phages that are incapable of lysogeny should be used for phage therapy [15]. Well-characterized phages that meet these criteria are often deposited in phage banks, serving as valuable resources for therapeutic or industrial applications [16].
Recently, we isolated and characterized two bacteriophages with broad host specificity against a panel of local uropathogenic E. coli strains [17]. In the present study, we contributed further to the establishment of a national phage bank by characterizing additional phages covering a broader spectrum of E. coli strains. Six phages with desirable properties were combined into a phage cocktail, and its antibacterial activity was measured in liquid artificial urine medium.
Materials and methods
Bacterial strains and culture conditions
In total, 76 E. coli isolates obtained from the urine of ambulatory or hospitalized patients with symptomatic bacteriuria [17] and reference E. coli strain CFT073 [18] were used in the study. Strains were classified into phylogenetic groups by quadruplex PCR [19] and into CH types by two-locus Sanger sequencing [20], and some strains were also analyzed by whole-genome sequencing [21]. Bacteria were cultivated overnight at 37°C on stationary Luria-Bertani (LB) plates or in LB broth with shaking at 220 rpm.
Bacteriophage isolation and propagation
Novel bacteriophages were isolated from wastewater or surface water by procedures that have been described previously [17]. Briefly, 10 ml of water was sterilized by passage through a 0.22-µm filter and mixed with an equal volume of twofold-concentrated LB medium supplemented with 0.5% glucose, 2.5 mM CaCl2, and 2.5 mM MgCl2 and 200 µL of overnight bacterial culture. The inoculated mixture was cultivated overnight at 37°C with shaking. The phage lysate was sterilized by adding 100 µL chloroform, and bacteria were removed by centrifugation at 9000 rpm for 30 min. Phages were purified through three repeated isolations from single plaques on double agar and then enriched by infecting an exponentially growing indicator strain at a multiplicity of infection (MOI) of 0.001, followed by overnight cultivation. The medium was removed, and phage particles were concentrated by polyethylene glycol (PEG) precipitation (10% PEG 600; 1 M NaCl) and resuspended in SM buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris-HCl, pH 7.5, and 0.002% gelatin) for long-term storage at 4°C. Prior to DNA isolation, phages were purified by cesium chloride gradient centrifugation, using 1.1, 1.3, and 1.5 mg/ml CsCl solutions. After 3 hours of centrifugation at 22,000 rpm at 10°C, a light blue phage band was transferred to dialysis tubing with a molecular weight cutoff of 10 kDa and dialyzed against SM buffer to remove excess salt. Two previously described phages (vKMB22 and vKMB26) [17] were also included in the present study.
Phage and bacterial genome sequencing and analysis
Phage and bacterial DNA were isolated using a Norgen Phage DNA Isolation Kit and a Canvas DNA Isolation Kit, respectively. Bacterial and phage genome libraries were prepared using a Nextera XT Library Preparation Kit according to a standard protocol. Paired-end 2×300-bp libraries were sequenced on an Illumina MiSeq platform. Reads were deduplicated and assembled de novo using the SPAdes 3.10.1 genome assembler. Annotation of genomes was done using the RASTtk server [22]. The functions of hypothetical proteins were predicted using HHpred [23]. Bacteriophages were classified using the Patric Taxonomy Classification Service [24], which uses k-mer matches, and were subsequently subjected to genome zeroing based on a reference phage of a given genus (ICTV taxonomy). Visualization and comparison of phage genomes was done in EasyFig 2.2.5 and in Geneious 11.1.5 (https://www.geneious.com). Nucleotide similarity (NS) of genomes was calculated by using VIRIDIC [25]. The web-based programs VirulenceFinder [26] and ResFinder [27] were used to find bacterial resistance and virulence genes in phage genomes. Lysogenic traits such as repressor, recombinase, and attachment sites were browsed manually in phage annotations and with the aid of the web-based program Phaster [28]. Predicted ORFs with known protein homologs were divided into five groups: structural proteins, enzymes and proteins for DNA replication/modification/regulation, packaging proteins, host lysis proteins, and additional proteins. The sequences of the phages were deposited in the GenBank database under the accession numbers shown in Table 1.
Determination of phage host range, growth curve, and phage adsorption
The susceptibility of bacterial isolates to the phages and the efficiency of plating (EOP) were tested by dropping 10 µl of phage dilutions in SM buffer onto double-layer agar plates. A strain was considered susceptible if single plaques were formed. The relative EOP of a phage was defined as strong, moderate, and weak when single plaques were observed at a concentration of 104, 106, and 108 plaque-forming units (PFU)/ml, respectively. An infection was considered abortive if a lysis zone appeared without plaque formation. The phage host range test was repeated twice.
A one-step growth curve was generated by adding 100 µl of 107 PFU/ml phage lysate to 10 ml of exponentially growing bacterial culture. Phages were allowed to adsorb for 10 min at 37°C, after which the mixture was then centrifuged and the pellet was resuspended in 10 ml of prewarmed LB. At this time, 1-ml samples were taken every 5 min, and the phages were counted by plaque assay.
Phage adsorption was measured by adding 20 µl of 108 PFU/ml phage suspension to 180 µl of overnight bacterial culture and allowing the phage to adsorb for 5 min at 37°C. Subsequently, 10 µl of sample was diluted in 1 ml of SM buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris-HCl, pH 7.5, and 0.002% gelatin), adsorbed phages were removed by centrifugation, and unadsorbed phages were counted by plaque assay. The measurements were repeated in triplicate.
Effect of phage cocktail on bacterial culture in liquid medium
Artificial urine (pH 6.5) was prepared as described previously [29] with some modifications. In order to avoid precipitation, the concentration of ureic acid was reduced to 0.4 mM, and to support bacterial growth, 0.1% peptone and 0.01% yeast extract were included [30]. An overnight bacterial culture was washed with PBS, diluted in LB medium or artificial urine to an OD580 of 0.1 (approx. 8 × 106 colony-forming units (CFU)/ml). Phage cocktails composed of six phages (Table 2) with concentrations of 3 × 109 PFU/ml (MOI = 100), 3 × 108 PFU/ml (MOI = 10), and 3 × 107 PFU/ml (MOI = 1) of each phage were prepared in PBS. An aliquot of 190 µl of bacterial culture and 10 µl of the phage cocktail were added to each well of a 96-well microtiter plate, and the inhibitory effect of the cocktail was evaluated by monitoring the optical density (OD580) in a Varioskan reader (Thermo Fisher) during 24 h of incubation at 37°C with shaking at 220 rpm. Each sample was tested in triplicate in three independent experiments (n = 9). The percentage of bacterial growth inhibition was calculated as the total area under the curve of the positive control minus the total area under the curve of the sample with phage cocktail, divided by the total area under the curve of the positive control × 100 [31]. The results are reported as the average relative bacterial growth inhibition from three experiments.
Results
Phage isolation and host specificity
In the present study, sixteen different bacteriophages infecting UPEC were isolated from sewage or surface water and characterized (Table 1). The phage host specificity was tested on a collection of 77 clinical E. coli strains belonging to different phylogenetic lineages and sequence types. The phages were able to infect 2–44 (3–57%) of the strains (Fig. 1). The broadest host specificity was observed for the phages vKMB26 and vKMB43, which lysed 44 and 33 (57 and 43%) of the strains, respectively. Sixty-two strains (80%) were infected by at least one phage, and E. coli KMB-735, the most sensitive strain, was infected by 12 different phages (Fig. 1). Susceptible E. coli strains belonged predominantly to the B2 phylogenetic group, including strains of clones CC131 and CC73, which have spread worldwide, and all but one strain of this group were susceptible to at least one phage (Fig. 1A). High sensitivity was also observed in strains belonging to phylogenetic group D (89% of the susceptible strains). Limited phage sensitivity was observed with strains of the A and B1 phylogroups, with 35% on average being vulnerable to at least one phage. One strain belonging to phylogenetic group C was resistant to all of the phages.
Genome sequencing of bacteriophages
Phage genome sequences were assembled de novo with a high level of contig coverage (> 300). All of the phages possessed a dsDNA genome and belonged to the class Caudoviricetes. The majority of them were classified as members of the family Straboviridae (T4-like phages) (n = 8), and the others belonged to the families Autographiviridae (n = 2) and Drexlerviridae (n = 2) and the genera Kagunavirus (n = 2), Justusliebigvirus (n = 1), and Murrayvirus (n = 1).
Genome sequence alignment to the reference phages of a given genus showed a high level of sequence similarity. However, based on the > 95% sequence identity cutoff for species demarcation by the ICTV [32], most of these phage isolates should be classified as members of new species (Table 1). Phages vKMB26 (genus Tequatrovirus) and vKMB47 (genus Vectrevirus) were the exceptions, showing 97% and 98% sequence identity to phages vB_EcoM-G3G7 (MZ234040.1) and vB_EcoP-101101UKE1 (MZ234012.1), respectively. The highest variability in the phage genomes was displayed in genes associated with receptor recognition. Except for phage vKMB25 (genus Tlsvirus), all of the phages were strictly lytic, and none of them encoded bacterial resistance or virulence genes.
Family Straboviridae
The eight novel Straboviridae phages in the collection exhibited the most extensive host range. Individually, these phages infected 17–56% of the UPEC strains, and collectively, they infected 73% of them. These novel phages were classified into three genera. Six phages were categorized as members of the genus Tequatrovirus, sharing a genome organization similar to those of T-even phages, with 83–86% nucleotide sequence identity to Escherichia phage T4 (NC_000866.4) (Fig. 2). While these six phages exhibited similarities in their genetic makeup, notable differences were observed in the sequences of the gp36, gp37, and gp38 long tail fiber proteins, the gp12 short tail fiber protein, and the RNA polymerase and the ADP ribosyltransferase, and they differed in the presence of homing endonuclease genes and genes with unknown functions. The Tequatrovirus phages formed three groups based on the organization of their long tail fiber genes and the similarity of their host range. The first group comprised vKMB22 and vKMB23, while the second group consisted of three phages: vKMB39, vKMB40, and vKMB42. vKMB26, which displayed the broadest host range within the entire collection (58%), was the most distantly related.
Another member of the family Straboviridae, phage vKMB38, was classified as a member of the genus Mosigvirus and exhibited 94% sequence identity to phage RB69 (NC_004928.1) (Fig. 2). Significant differences between RB69 were observed in the dihydrofolate reductase gene frd (34% identity), the capsid decoration protein gene hoc (29% identity), and the receptor binding protein gp37 gene (70% identity). Despite possessing promising characteristics typically associated with highly virulent phages, vKMB38 demonstrated a narrower host range, being able to infect only 17% of the tested UPEC strains. Of the Straboviridae family members tested, it had the most limited host range.
Phage vKMB43 displayed a close relationship to the pseudo-T-even krischvirus RB49 (NC_005066.1), with 92% nucleotide sequence identity (Fig. 2). Unlike the other members of the family Straboviridae, vKMB43 lacks any tRNA genes, which is a characteristic feature of members of the genus Krischvirus. Notably, vKMB43 demonstrated a wide host range, infecting 43% of the tested strains, predominantly within the B1, B2, and D phylogroups.
Genus Justusliebigvirus
Phage vKMB37 has a 148-kbp-long genome and is related to members of the genus Justusliebigvirus, namely phi92 (NC_023693.1) (Fig. 2), Escherichia phage inny (MN850601.1), and VEcB (NC_052663.1). These phages represent a distinct branch within the order Caudoviricetes and are placed within the subfamily Stephanstirmvirinae. Like other Justusliebigvirus phages, vKMB37 contains numerous tRNA genes (12) and several tail spike proteins. However, vKMB37 differs from phi92 in that the K1-specific neuraminidase is replaced by another putative glycosidase in vKMB37. This phage was able to infect 23% of the tested strains, primarily within the B1, B2, and D phylogroups.
Genus Kagunavirus
Two phages, vKMB35 and vKMB36, were identified as members of the genus Kagunavirus within the subfamily Guernseyvirinae. These phages have collinear genomes approximately 44 kbp in length, displaying low nucleotide sequence similarity to each other as well as to the reference phage K1G (NC_027993.1) (64% identity). Notably, the genomes of these phages exhibited a high degree of mosaicism, with regions of notable similarity being interrupted by non-similar homologous genes (Fig. 2). Both vKMB35 and vKMB36 displayed a narrow host range, with vKMB35 infecting six (8%) strains, while vKMB36 infected only two (3%) strains belonging to the B2 phylogroup.
Genus Vectrevirus
Two phages, vKMB14 and vKMB47, were identified as members of the genus Vectrevirus within the family Autographiviridae. These phages possess 44-kbp genomes exhibiting a typical T7-like organization. Their genome sequences are 89% identical to each other and to the reference phage VEc3 (NC_047899.1) (Fig. 2). The genomes of vKMB14 and vKMB47 differ primarily in the tail spike region. Notably, both phages form clear plaques with halo zones, suggesting the presence of a polysaccharide depolymerase in the phage receptor binding protein. In terms of host range, vKMB14 and vKMB47 were able to infect 17% and 22% of the tested strains, respectively. The host spectrum partially overlapped, with the sensitive strains predominantly belonging to the B2 phylogroup.
Family Drexlerviridae
Two of the phages belonged to the family Drexlerviridae. vKMB25, with a 50-kbp genome, was found to be highly similar to the tlsvirus Stevie (NC_027350.1, 90.5% identity, Fig. 2). We found some features of vKMB25 that could be associated with temperate phages, one of them being a potential prophage insertion site (attL/attR) with the nucleotide sequence GTTGACGGCGTG. vKMB25 was also found to encode a homolog of the superinfection exclusion Cor lipoprotein, which protects a lysogenized host from superinfection by inactivating the phage receptor. Despite the fact that no repressor or integrase genes were detected, there is a chance that vKMB25 could be a temperate phage and thus unsuitable for therapeutic applications. Moreover, its host range was found to be low, infecting only 5% of the tested strains.
Another member of this family, vKMB46, was highly similar to swan01 phage (genus Warwickvirus) (NC_048202.1), with 92% nucleotide sequence identity and a similar genome length of 50 kbp (Fig. 2). Most of the predicted proteins, especially in the 18 kbp-region upstream of the terminase gene, had no assigned function, even after re-annotation using HHpred. The vKMB46 structural genes are organized similarly to those of the temperate phage lambda (NC_001416.1), although the genes required for a lysogenic cycle were not present. Phage vKMB46 infected 10% of the strains tested.
Genus Murrayvirus
Phage vKMB41 was identified as a member of the genus Murrayvirus. The reference phage EC2 (genus Murrayvirus; NC_047742.1) shared 73% sequence identity with vKMB41 (Fig. 2). Phages of this genus typically have a relatively small genome (approx. 42 kbp) and high GC content (> 59%). vKMB41 was found to have narrow host specificity, infecting 5% of the tested strains.
Inhibition of E. coli growth by a phage cocktail
Based on their host range, genome features, and growth properties, a phage cocktail composed of six phages – four Straboviridae members and two Autographiviridae members – was prepared (Table 2). Altogether, these phages were able to productively infect 52 (67%) of the tested strains. The selected phages had a high adsorption rate (72–98% in 5 min), a short latent period (7.5–15 min), and relatively high burst size (38–211) when measured on the propagating strain (Table 2). The efficiency of the cocktail was tested on a panel of seven E. coli strains belonging to frequently detected UPEC clones. Double agar plate testing showed that the phages were highly infectious for six strains, as these strains were productively infected by 3–6 phages from the cocktail. In addition, E. coli KMB-704 (ST69), a strain that is resistant to almost all phages, was also included in the assay.
Inhibition of E. coli growth was measured in microtiter plates for 24 hours using two media: rich LB and artificial urine medium (AU). We observed great variations in the degree of bacterial growth inhibition by the phage cocktail. In six susceptible strains, almost complete suppression of bacterial growth was observed in the first 5–22 hours of incubation. After this period, the optical density started to increase but did not reach the level of the positive control (Fig. 3). No significant difference between cocktails with an EOP of 1, 10, and 100 was observed. Overgrowth of the culture did not correlate with the number of phages to which the strain was susceptible. Instead, it appeared to be a random process, and for some strains (especially E. coli KMB-735), great variations between parallel growth experiments were observed. Some differences in cocktail efficiency between nutrient-rich LB medium and artificial urine medium were also observed. Overall, the level of inhibition of six susceptible strains by the cocktail at 24 hours postinfection reached 43–92% in LB medium and 53–79% in AU medium (Fig. 4). In AU medium, similar levels of inhibition were observed for strains that were pre-adapted by overnight cultivation in AU medium as for LB-grown inoculum (data not shown). In the case of the phage-resistant strain E. coli KMB-704 (ST69), the phage cocktail did not affect its growth, although the optical density decreased slightly during late phase of cultivation in LB medium, with an overall reduction of 76%.
Bacterial growth curves in the presence of phage cocktail in LB and AU media. Growth inhibition was observed as a decrease in optical density compared to control without phages. Three different MOIs were used: 1 (triangle), 10 (square), 100 (diamond), and none in control (circle). Results are presented as the mean of three measurements replicated in three independent experiments
Overall bacterial growth inhibition in the presence of phage cocktail in LB and AU media. Growth inhibition was observed as an area of optical density in cocktail samples divided by an area of optical density in control samples without phages. Three different MOIs were used: 1 (white), 10 (grey), and 100 (black)
Discussion
Phages for phage therapy applications can be easily isolated from the environment [12, 33, 34]. However, not every phage is suitable for therapy. Only phages with an appropriate host range should be used, and this may vary depending on the country or environment, as pathogenic strains are adapted to local conditions. It is also necessary to use strictly lytic phages that do not have genes encoding virulence factors or conferring antibiotic resistance. Such well-characterized phages are deposited in phage banks, which serve as a source of phages for therapeutic or industrial applications [16, 34].
In the present study, we characterized 16 coliphages isolated from wastewater as candidates for UPEC control agents. Two of the phages, vKMB22 and vKMB26, which were isolated and partially characterized in our previous study [17], were also included. The phages showed a variable range of host specificity on a panel of UPEC strains (Fig. 1). The broadest host specificity was observed for phages belonging to the family Straboviridae. The Autographiviridae and Stephanstirmvirinae members were able to infect a moderate number of hosts, and other phages possessed narrow specificity. This observation is consistent with the fact that Straboviridae (T-even) phages are among the most frequently isolated E. coli phages and are used in various applications [35,36,37,38]. However, we also observed up to twofold differences in the host range between closely related Tequatrovirus phages. Phage sensitivity is influenced by many factors. For example, a point mutation in the phage adhesin has been reported to cause a substantial decrease in the host spectrum of Straboviridae phage Pet-CM3-4 infecting members of the genera Cronobacter and Enterobacter [39].
We observed that the E. coli strains that were susceptible to the phages in this study belonged predominantly to the phylogenetic groups B2 and D. Limited phage sensitivity was observed with strains of phylogroups A and B1 (Fig. 1A). Such preferences for certain E. coli phylogenetic groups were observed previously in phages isolated from poultry meat or feces, which predominantly infected members of phylogroup A, B1, or D [40]. Similarly, phages isolated from wastewater in Germany showed increased virulence against phylogroup B2 [38]. It is not obvious why we were unable to isolate phages with a broad host range within phylogroups A or B1 despite the fact that six of the phages were initially propagated on indicator strains of these phylogroups and some of them showed moderate activity against B2 strains. In general, the habitat of a bacteriophage is dependent on its host bacteria, and our results might reflect the predominance of B2 strains among E. coli in wastewater [5, 7].
Genome analysis of the phages revealed that they all belonged to known genera, but most of them did not belong to established species, as they differed at > 95% of their nucleotide positions from phages with sequences in the GenBank database [32]. Phages vKMB26 (genus Tequatrovirus) and vKMB47 (genus Vectrevirus) were the exceptions, as they were almost identical to phages isolated recently from wastewater in Germany [38]. Those phages, like the ones in this study, exhibited a broad host range on UPEC strains. Isolation of phages of the same species in different countries implies that the global spread of UPEC strains, such as sequence type ST131 [5, 21], has favored the distribution of phages adapted to them.
A comparison of the genome sequences of the Straboviridae phages revealed mosaicism, which is a typical feature of the T-even group (Fig. 2) [41]. Many of these variable genes are responsible for overcoming host defenses, such as the dCMP hydroxymethylases, glucosyl transferases, and orphan DAM methylases, which are responsible for extensive phage DNA modifications, and internal head proteins (IP) conferring protection against GmrSD endonuclease [42, 43]. The genome of phage vKMB26 encodes an extra beta-glucosyltransferase gene with no homology to previously characterized genes, which could provide the phage with an unusual DNA glucoslylation pattern. This modification, together with a unique sequence of distal tail fibers, may explain the extended host range of vKMB26.
Phage vKMB38 (genus Mosigvirus) similarly to reference phage RB69, does not encode a glucosyltransferase, suggesting the presence of hmC without glucosylation. However, a putative glycosyltransferase responsible for arabinosyl-hmC modification in phage RB69 [44] was also found in vKMB38. Despite some properties that are typical of highly virulent phages (e.g., the presence of a dmd antitoxin gene), vKMB38 could infect only 17% of the UPEC strains tested and is therefore the member of the family Straboviridae with narrowest host range in this study.
Two phages of the genus Vectrevirus, family Autographiviridae, vKMB14 and vKMB47, have a typical T7-like organization and differ mainly in the tail spike region, and this might account for differences in their host spectrum. In both genomes, we identified two tail spike genes encoding polysaccharide depolymerases with different specificity. Such an arrangement is frequently found in Autographiviridae phages [45]. The first spike in both genomes has a high degree of similarity to the K5 lyase of Escherichia phage K1-5. The second tail spike in vKMB14 shows similarity to the pectate lyase domain [46], but with low amino acid sequence similarity to other phage tail spikes (< 43%), giving it unique specificity. The other phage, vKMB47, was found to be highly similar to the recently described Escherichia phage vB_EcoP-101101UKE1 [38, 46], and a high level of similarity was also found in the second tail spike gene.
The remaining six phages were classified into five genera, possessed lower host specificity, and belonged to taxa that are more rarely represented in DNA databases. Of these, phage vKMB37 (genus Justusliebigvirus) was able to infect the largest number of strains. This phage is closely related to phage phi92 [47], but it contains a different RBP gene. As vKMB37 infects strains with different types of capsular polysaccharide, it seems that it is not as capsule-dependent as phi92. Like phi92, vKMB37 carries alx-like ter cluster genes, which are involved in bacterial stress response systems [47, 48]. The function of these genes during the phage life cycle is unknown.
The two phage isolates belonging to the genus Kagunavirus, vKMB35 and vKMB36, were found to be distantly related to each other (Fig. 2). Both of these produced plaques with turbid halos and encoded polysaccharide depolymerases that did not show amino acid sequence similarity to the sialidase of K1G or to any other depolymerases. It is interesting that vKMB36 seems to contain a 900-bp intein in the minor head protein gene. Inteins are mostly found in DNA-binding proteins, such as terminase [49], and they were also detected recently in the capsid proteins of Salmonella phages [49, 50]. Surprisingly, there are no reports of inteins in E. coli or in coliphages, suggesting that vKMB36 obtained the intein from another host, probably from Salmonella.
Overall, we can conclude that isolated phages, except for vKMB25, possess genome properties suitable for therapeutic use, lacking known virulence or antibiotic resistance genes.
Based on our previous results, the six phages with the best properties were combined into a phage cocktail, the antibacterial activity of which was measured in vitro. Phage cocktails represent the best way to prepare phage preparations with sufficient host specificity [40, 51]. In most cases, the selection of phages for a cocktail is based on their host specificity and growth parameters, based on empirical rules [52]. Four Straboviridae and two Autographiviridae phages were combined for this study. These phages belong to the species found most frequently in the environment and whose members have been used in previous phage therapy studies [51, 53]. The two Autographiviridae phages are predicted to produce polysaccharide depolymerases, which could potentially decompose bacterial capsules or biofilms, and thus could improve the efficiency of the cocktail. The phages selected to the cocktail possessed broad host specificity on double agar and excellent growth properties when measured on the original propagating strain (Table 2).
When the activity of the cocktail was tested in liquid medium we did not observe bacterial growth during the first hours of cultivation, but partial regrowth was observed at later times (Fig. 3). Development of phage resistance did not correlate with the number of phages infecting a particular strain on double agar but instead appears to have been a random process, and for some strains, great variations between parallel experiments were also observed. Interestingly, the highest growth suppression was observed for two strains, KMB-706 and KMB-507, which were sensitive only to phages of the family Straboviridae. In the present study, bacterial receptors were not identified, and it is therefore possible that multiple phages in the cocktail bind to the same receptor, making co-selection of resistance to these phages possible. Our observations are consistent with a previous report that the spot method tends to indicate greater phage sensitivity than the microtiter assay, which is better for monitoring host-phage interactions [31].
The cocktail prepared in this study was efficient in LB as well as in moderately acidic artificial urine medium, which mimics conditions present in UTIs [29, 30]. It is interesting that, in some cases, the efficiency of the cocktail in artificial urine was even better than in LB. These results indicate that our phage cocktail has the potential to inhibit bacteria during infection, and these phage isolates will therefore be preserved in the national phage bank, serving as valuable resources for therapeutic applications.
Data Availability
DNA sequences were deposited in GenBank, the other data are presented in the manuscript.
References
Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284
Foxman B (2010) The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660
Terlizzi ME, Gribaudo G, Maffei ME (2017) UroPathogenic (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front Microbiol 8:1566
Ejrnæs K, Stegger M, Reisner A, Ferry S, Monsen T, Holm SE et al (2011) Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: Phylogenetic groups, virulence factors and biofilm formation. Virulence 2:528–537. https://doi.org/10.4161/viru.2.6.18189
Hyun M, Lee JY, Kim HA (2022) Comparison of the Clinical and Genotypic Characteristics of Uropathogenic Strains According to Sex in Korea. Microb Drug Resist 28:988–996
Manges AR, Tabor H, Tellis P, Vincent C, Tellier P-P (2008) Endemic and Epidemic Lineages of Escherichia coli that Cause Urinary Tract Infections. Emerg Infect Dis 14:1575–1583
Meziani DY, Barnich N, Boucheham A, Rezgoune ML, Benlabed K, Rodrigues M et al (2022) Identification of virulence markers and phylogenetic groups association, and Antimicrobial Susceptibility of uropathogenic Escherichia coli isolates. Infect Disord Drug Targets 23:e080922208695
Pitout JDD, DeVinney R (2017) Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000 Res 6:195
Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R et al (2016) Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. MBio 7:e02162
Mathers AJ, Peirano G, Pitout JDD (2015) Escherichia coli ST131: The quintessential example of an international multiresistant high-risk clone. Adv Appl Microbiol 90:109–154
Chen Y-H, Ko W-C, Hsueh P-R (2013) Emerging resistance problems and future perspectives in pharmacotherapy for complicated urinary tract infections. Expert Opin Pharmacother 14:587–596
Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (2011) Phage treatment of human infections. Bacteriophage 1:66–85
Roach DR, Debarbieux L (2017) Phage therapy: awakening a sleeping giant. Emerg Top Life Sci 1:93–103
Bao J, Wu N, Zeng Y, Chen L, Li L, Yang L et al (2020) Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant. Emerg Microbes Infect 9:771–774
Knoll BM, Mylonakis E (2014) Antibacterial bioagents based on principles of bacteriophage biology: an overview. Clin Infect Dis 58:528–534
Lin RC, Sacher JC, Ceyssens P-J, Zheng J, Khalid A, Iredell JR et al (2021) Phage Biobank: Present Challenges and Future Perspectives. Curr Opin Biotechnol 68:221–230
Slobodníková L, Markusková B, Kajsík M, Andrezál M, Straka M, Liptáková A et al (2021) Characterization of Anti-Bacterial Effect of the Two New Phages against Uropathogenic Escherichia coli. Viruses 13:1348
Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV et al (1990) Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1289
Clermont O, Christenson JK, Denamur E, Gordon DM (2013) The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. : 58–65
Ahmed S, Besser TE, Call DR, Weissman SJ, Jones LP, Davis MA (2016) Evaluation of two multi-locus sequence typing schemes for commensal Escherichia coli from dairy cattle in Washington State. J Microbiol Methods. : 57–61
Koreň J, Andrezál M, Ozaee E, Drahovská H, Wawruch M, Liptáková A et al (2023) High Emergence of Multidrug-Resistant Sequence Type 131 Subclade C2 among Extended-Spectrum β-Lactamase (ESBL)-Producing Isolated from the University Hospital Bratislava. Slovakia Antibiot 12:1209
Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ et al (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365
Hildebrand A, Remmert M, Biegert A, Söding J (2009) Fast and accurate automatic structure prediction with HHpred. Proteins 77(Suppl 9):128–132
Davis JJ, Wattam AR, Aziz RK, Brettin T, Butler R, Butler RM et al (2020) The PATRIC Bioinformatics Resource Center: expanding data and analysis capabilities. Nucleic Acids Res 48:D606–D612
Moraru C, Varsani A, Kropinski AM (2020) VIRIDIC-A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 12:1268
Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM et al (2014) Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol 52:1501–1510
Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V et al (2020) ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother 75:3491–3500
Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y et al (2016) PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res 44:W16–21
Sarigul N, Korkmaz F, Kurultak İ (2019) A New Artificial Urine Protocol to Better Imitate Human Urine. Sci Rep 9:20159
Brooks T, Keevil CW (1997) A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol 24:203–206
Xie Y, Wahab L, Gill JJ (2018) Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 10:189
Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Adriaenssens EM, Dempsey DM et al (2020) Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of. Viruses Arch Virol 165:2737–2748
Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S et al (2010) Phage Therapy in Clinical Practice: Treatment of Human Infections. Curr Pharmaceut Biotechnol 11:69–86. https://doi.org/10.2174/138920110790725401
Gelman D, Yerushalmy O, Alkalay-Oren S, Rakov C, Ben-Porat S, Khalifa L et al (2021) Clinical Phage Microbiology: a suggested framework and recommendations for the in-vitro matching steps of phage therapy. Lancet Microbe 2:e555–e563
Grose JH, Casjens SR (2014) Understanding the enormous diversity of bacteriophages: the tailed phages that infect the bacterial family Enterobacteriaceae. Virology 468–470:421–443
Śliwka P, Weber-Dąbrowska B, Żaczek M, Kuźmińska-Bajor M, Dusza I, Skaradzińska A (2023) Characterization and Comparative Genomic Analysis of Three Virulent Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment. Int J Mol Sci 24:5696
Pham-Khanh NH, Sunahara H, Yamadeya H, Sakai M, Nakayama T, Yamamoto H, Truong Thi Bich V, Miyanaga K, Kamei K (2019) Isolation, characterisation and complete genome sequence of a Tequatrovirus phage, Escherichia phage KIT03, which simultaneously infects Escherichia coli O157:H7 and Salmonella enterica. Curr Microbiol 76:1130–1137
Loose M, Sáez Moreno D, Mutti M, Hitzenhammer E, Visram Z, Dippel D et al (2021) Natural Bred ε-Phages Have an Improved Host Range and Virulence against Uropathogenic over Their Ancestor Phages. Antibiotics 10:1337
Andrezal M, Oravcova L, Kadličekova V, Ozaee E, Elnwrani S, Bugala J et al (2023) Characterization and the host specificity of Pet-CM3-4, a new phage infecting Cronobacter and Enterobacter strains. Virus Res 324:199025
Kim J, Park H, Ryu S, Jeon B (2021) Inhibition of Antimicrobial-Resistant Using a Broad Host Range Phage Cocktail Targeting Various Bacterial Phylogenetic Groups. Front Microbiol 12:699630
Comeau AM, Bertrand C, Letarov A, Tétart F, Krisch HM (2007) Modular architecture of the T4 phage superfamily: a conserved core genome and a plastic periphery. Virology 362:384–396
Bair CL, Rifat D, Black LW (2007) Exclusion of glucosyl-hydroxymethylcytosine DNA containing bacteriophages is overcome by the injected protein inhibitor IPI*. J Mol Biol 366:779–789
Petrov VM, Ratnayaka S, Nolan JM, Miller ES, Karam JD (2010) Genomes of the T4-related bacteriophages as windows on microbial genome evolution. Virol J 7:292
Thomas JA, Orwenyo J, Wang L-X, Black LW (2018) The Odd RB Phage-Identification of Arabinosylation as a New Epigenetic Modification of DNA in T4-Like Phage RB69. Viruses 10:313
Latka A, Leiman PG, Drulis-Kawa Z, Briers Y (2019) Modeling the Architecture of Depolymerase-Containing Receptor Binding Proteins in Phages. Front Microbiol 10:2649
Xiang Y, Leiman PG, Li L, Grimes S, Anderson DL, Rossmann MG (2009) Crystallographic insights into the autocatalytic assembly mechanism of a bacteriophage tail spike. Mol Cell 34:375–386
Schwarzer D, Buettner FFR, Browning C, Nazarov S, Rabsch W, Bethe A et al (2012) A multivalent adsorption apparatus explains the broad host range of phage phi92: a comprehensive genomic and structural analysis. J Virol 86:10384–10398
Anantharaman V, Iyer LM, Aravind L (2012) Ter-dependent stress response systems: novel pathways related to metal sensing, production of a nucleoside-like metabolite, and DNA-processing. Mol Biosyst 8:3142–3165
Novikova O, Jayachandran P, Kelley DS, Morton Z, Merwin S, Topilina NI et al (2016) Intein Clustering Suggests Functional Importance in Different Domains of Life. Mol Biol Evol 33:783–799
Kuźmińska-Bajor M, Śliwka P, Ugorski M, Korzeniowski P, Skaradzińska A, Kuczkowski M et al (2021) Genomic and functional characterization of five novel Salmonella-targeting bacteriophages. Virol J 18:183
Petrovic Fabijan A, Iredell J, Danis-Wlodarczyk K, Kebriaei R, Abedon ST (2023) Translating phage therapy into the clinic: Recent accomplishments but continuing challenges. PLoS Biol 21:e3002119
Lood C, Haas P-J, van Noort V, Lavigne R (2022) Shopping for phages? Unpacking design rules for therapeutic phage cocktails. Curr Opin Virol 52:236–243
Gibson SB, Green SI, Liu CG, Salazar KC, Clark JR, Terwilliger AL et al (2019) Constructing and Characterizing Bacteriophage Libraries for Phage Therapy of Human Infections. Front Microbiol 10:2537
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This work was supported by Slovak Research and Development Agency under the contracts No. APVV-16-0168 and APVV-20-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Johannes Wittmann
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markusková, B., Elnwrani, S., Andrezál, M. et al. Characterization of bacteriophages infecting multidrug-resistant uropathogenic Escherichia coli strains. Arch Virol 169, 142 (2024). https://doi.org/10.1007/s00705-024-06063-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-024-06063-x