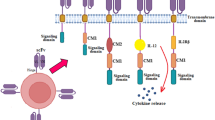
Abstract
T cell immunoglobulin and mucin domain containing protein 3 (Tim-3), an immune checkpoint, is important for maintaining immune tolerance. There is increasing evidence that Tim-3 is aberrantly expressed in patients with COVID-19, indicating that it may play an important role in COVID-19. In this review, we discuss the altered expression and potential role of Tim-3 in COVID-19. The expression of Tim-3 and its soluble form (sTim-3) has been found to be upregulated in COVID-19 patients. The levels of Tim-3 on T cells and circulating sTim-3 have been shown to be associated with the severity of COVID-19, suggesting that this protein could be a potential biomarker of COVID-19. Moreover, this review also highlights the potential of Tim-3 as a therapeutic target of COVID-19.





Similar content being viewed by others
References
Das M, Zhu C, Kuchroo VK (2017) Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 276(1):97–111. https://doi.org/10.1111/imr.12520
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415(6871):536–541. https://doi.org/10.1038/415536a
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44(5):989–1004. https://doi.org/10.1016/j.immuni.2016.05.001
Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK (2005) The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med 11(8):362–369. https://doi.org/10.1016/j.molmed.2005.06.008
Tsai HF, Hsu PN (2017) Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci 24(1):35. https://doi.org/10.1186/s12929-017-0341-0
Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK (2003) Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 4(11):1102–1110. https://doi.org/10.1038/ni988
Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 4(11):1093–1101. https://doi.org/10.1038/ni987
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, Huang M, Zhou J (2013) Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS ONE 8(1):e53834. https://doi.org/10.1371/journal.pone.0053834
Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R (2010) Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 107(33):14733–14738. https://doi.org/10.1073/pnas.1009731107
Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z (2012) Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol 42(5):1180–1191. https://doi.org/10.1002/eji.201141852
Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK (2012) Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE 7(10):e47648. https://doi.org/10.1371/journal.pone.0047648
McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR (2010) Tim-3 expression on PD-1 + HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest 120(12):4546–4557. https://doi.org/10.1172/JCI43127
Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR (2009) Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4 + and CD8 + T cells. J Virol 83(18):9122–9130. https://doi.org/10.1128/JVI.00639-09
Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205(12):2763–2779. https://doi.org/10.1084/jem.20081398
Wang C, Horby PW, Hayden FG, Gao GF (2020a) A novel coronavirus outbreak of global health concern. Lancet (London England) 395(10223):470–473. https://doi.org/10.1016/S0140-6736(20)30185-9
Shekunov EV, Zlodeeva PD, Efimova SS, Muryleva AA, Zarubaev VV, Slita AV, Ostroumova OS (2023) Cyclic lipopeptides as membrane fusion inhibitors against SARS-CoV-2: New tricks for old dogs. Antiviral Res 212:105575. https://doi.org/10.1016/j.antiviral.2023.10557517
Paces J, Strizova Z, Smrz D, Cerny J (2020) COVID-19 and the immune system. Physiol Res 69(3):379–388. https://doi.org/10.33549/physiolres.934492
Barnova M, Bobcakova A, Urdova V, Kosturiak R, Kapustova L, Dobrota D, Jesenak M (2021) Inhibitory immune checkpoint molecules and exhaustion of T cells in COVID-19. Physiol Res 70(S2):S227–S247. https://doi.org/10.33549/physiolres.934757
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6(12):1245–1252. https://doi.org/10.1038/ni1271
Zhao L, Cheng S, Fan L, Zhang B, Xu S (2021) TIM-3: An update on immunotherapy. Int Immunopharmacol 99:107933. https://doi.org/10.1016/j.intimp.2021.107933
Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M (2003) Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol 170(7):3631–3636. https://doi.org/10.4049/jimmunol.170.7.3631
Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS (2015) CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517(7534):386–390. https://doi.org/10.1038/nature13848
Kandel S, Adhikary P, Li G, Cheng K (2021) The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett 510:67–78. https://doi.org/10.1016/j.canlet.2021.04.011
Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113(16):3821–3830. https://doi.org/10.1182/blood-2008-10-185884
Herrmann M, Schulte S, Wildner NH, Wittner M, Brehm TT, Ramharter M, Woost R, Lohse AW, Jacobs T, Schulze Zur Wiesch J (2020) Analysis of Co-inhibitory Receptor Expression in COVID-19 Infection Compared to Acute Plasmodium falciparum Malaria: LAG-3 and TIM-3 Correlate With T Cell Activation and Course of Disease. Front Immunol 11:1870. https://doi.org/10.3389/fimmu.2020.01870
Varchetta S, Mele D, Oliviero B, Mantovani S, Ludovisi S, Cerino A, Bruno R, Castelli A, Mosconi M, Vecchia M, Roda S, Sachs M, Klersy C, Mondelli MU (2021) Unique immunological profile in patients with COVID-19. Cell Mol Immunol 18(3):604–612. https://doi.org/10.1038/s41423-020-00557-9
Song JW, Zhang C, Fan X, Meng FP, Xu Z, Xia P, Cao WJ, Yang T, Dai XP, Wang SY, Xu RN, Jiang TJ, Li WG, Zhang DW, Zhao P, Shi M, Agrati C, Ippolito G, Maeurer M, Zumla A, Wang FS, Zhang JY (2020) Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 11(1):3410. https://doi.org/10.1038/s41467-020-17240-2
Shahbazi M, Moulana Z, Sepidarkish M, Bagherzadeh M, Rezanejad M, Mirzakhani M, Jafari M, Mohammadnia-Afrouzi M (2021) Pronounce expression of Tim-3 and CD39 but not PD1 defines CD8 T cells in critical Covid-19 patients. Microb Pathog 153:104779. https://doi.org/10.1016/j.micpath.2021.104779
Modabber Z, Shahbazi M, Akbari R, Bagherzadeh M, Firouzjahi A, Mohammadnia-Afrouzi M (2021) TIM-3 as a potential exhaustion marker in CD4(+) T cells of COVID-19 patients. Immun Inflamm Dis 9(4):1707–1715. https://doi.org/10.1002/iid3.526
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y (2020) Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 11:827. https://doi.org/10.3389/fimmu.2020.00827
Liu Y, Pan Y, Hu Z, Wu M, Wang C, Feng Z, Mao C, Tan Y, Liu Y, Chen L, Li M, Wang G, Yuan Z, Diao B, Wu Y, Chen Y (2020) Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells. Clin Infect diseases: official publication Infect Dis Soc Am 71(16):2150–2157. https://doi.org/10.1093/cid/ciaa630
Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L, Fang M, Zhang H, Sun Z (2020b) The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI insight 5(10). https://doi.org/10.1172/jci.insight.137799
Bobcakova A, Petriskova J, Vysehradsky R, Kocan I, Kapustova L, Barnova M, Diamant Z, Jesenak M (2021) Immune Profile in Patients With COVID-19: Lymphocytes Exhaustion Markers in Relationship to Clinical Outcome. Front Cell Infect Microbiol 11:646688. https://doi.org/10.3389/fcimb.2021.646688
Yang J, Chang T, Tang L, Deng H, Chen D, Luo J, Wu H, Tang T, Zhang C, Li Z, Dong L, Yang XP, Tang ZH (2022) Increased Expression of Tim-3 Is Associated With Depletion of NKT Cells In SARS-CoV-2 Infection. Front Immunol 13:796682. https://doi.org/10.3389/fimmu.2022.796682
Yao Y, Deng H, Li P, Zhang J, Zhang J, Wang D, Li S, Luo Y, Wei Z, Bi G, Yang XP, Tang ZH (2017) alpha-Lactose Improves the Survival of Septic Mice by Blockade of TIM-3 Signaling to Prevent NKT Cell Apoptosis and Attenuate Cytokine Storm. Shock 47(3):337–345. https://doi.org/10.1097/SHK.0000000000000717
Kreutmair S, Unger S, Nunez NG, Ingelfinger F, Alberti C, De Feo D, Krishnarajah S, Kauffmann M, Friebel E, Babaei S, Gaborit B, Lutz M, Jurado NP, Malek NP, Goepel S, Rosenberger P, Haberle HA, Ayoub I, Al-Hajj S, Nilsson J, Claassen M, Liblau R, Martin-Blondel G, Bitzer M, Roquilly A, Becher B (2021) Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity 54(7):1578–1593e5. https://doi.org/10.1016/j.immuni.2021.05.002
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A (2020) Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 27(6):992–1000e3. https://doi.org/10.1016/j.chom.2020.04.009
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory medicine 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Ueland T, Heggelund L, Lind A, Holten AR, Tonby K, Michelsen AE, Jenum S, Jorgensen MJ, Barratt-Due A, Skeie LG, Nordoy I, Aanensen Fraz MS, Quist-Paulsen EE, Pischke SE, Johal SK, Hesstvedt L, Bogen M, Fevang B, Halvorsen B, Muller F, Bekken GK, Mollnes TE, Dudman S, Aukrust P, Dyrhol-Riise AM, Holter JC (2021) Elevated plasma sTIM-3 levels in patients with severe COVID-19. J Allergy Clin Immunol 147(1):92–98. https://doi.org/10.1016/j.jaci.2020.09.007
Chavez-Galan L, Ruiz A, Martinez-Espinosa K, Aguilar-Duran H, Torres M, Falfan-Valencia R, Perez-Rubio G, Selman M, Buendia-Roldan I (2022) Circulating Levels of PD-L1, TIM-3 and MMP-7 Are Promising Biomarkers to Differentiate COVID-19 Patients That Require Invasive Mechanical Ventilation. Biomolecules 12(3). https://doi.org/10.3390/biom12030445
Zilber E, Martin GE, Willberg CB, Fox J, Nwokolo N, Fidler S, Frater J, Investigators C (2019) Soluble plasma programmed death 1 (PD-1) and Tim-3 in primary HIV infection. AIDS 33(7):1253–1256. https://doi.org/10.1097/QAD.0000000000002165
Wu H, He P, Ren Y, Xiao S, Wang W, Liu Z, Li H, Wang Z, Zhang D, Cai J, Zhou X, Jiang D, Fei X, Zhao L, Zhang H, Liu Z, Chen R, Li W, Wang C, Zhang S, Qin J, Nashan B, Sun C (2022) Postmortem high-dimensional immune profiling of severe COVID-19 patients reveals distinct patterns of immunosuppression and immunoactivation. Nat Commun 13(1):269. https://doi.org/10.1038/s41467-021-27723-5
Boussiotis VA (2016) Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 375(18):1767–1778. https://doi.org/10.1056/NEJMra1514296
Loretelli C, Abdelsalam A, D'Addio F, Ben Nasr M, Assi E, Usuelli V, Maestroni A, Seelam AJ, Ippolito E, Di Maggio S, Loreggian L, Radovanovic D, Vanetti C, Yang J, El Essawy B, Rossi A, Pastore I, Montefusco L, Lunati ME, Bolla AM, Biasin M, Antinori S, Santus P, Riva A, Zuccotti GV, Galli M, Rusconi S, Fiorina P (2021) PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response. JCI insight 6(24). https://doi.org/10.1172/jci.insight.146701
Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X, Deng L, Wang Y, Zhang X, Jiang T, Lu X (2016) Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. J immunotherapy (Hagerstown Md: 1997) 39(4):171–180. https://doi.org/10.1097/CJI.0000000000000122
Kang CK, Han GC, Kim M, Kim G, Shin HM, Song KH, Choe PG, Park WB, Kim ES, Kim HB, Kim NJ, Kim HR, Oh MD (2020) Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int J Infect diseases: IJID : official publication Int Soc Infect Dis 97:313–321. https://doi.org/10.1016/j.ijid.2020.05.106
Lovly CM, Boyd KL, Gonzalez-Ericsson PI, Lowe CL, Brown HM, Hoffman RD, Sterling BC, Kapp ME, Johnson DB, Kopparapu PR, Iams WT, Warren MA, Noto MJ, Rini BI, Jagasia M, Das SR, Balko JM (2020) Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv: the preprint server for health sciences. https://doi.org/10.1101/2020.04.29.20085738
Acknowledgements
We would like to thank the editor and anonymous reviewers for their insightful comments and constructive suggestions, which helped us to improve the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32000650) and the Natural Science Foundation of Henan Province (Grant No. 232300421292).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Roman Pogranichniy
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, H., Ren, S., Meng, Q. et al. Role of Tim-3 in COVID-19: a potential biomarker and therapeutic target. Arch Virol 168, 213 (2023). https://doi.org/10.1007/s00705-023-05842-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05842-2