Abstract
Dengue virus (DENV), a member of the genus Flavivirus, family Flaviviridae, is the most widespread viral pathogen transmitted to humans by mosquitoes. Despite the increased incidence of DENV infection, there are no antiviral drugs available for treatment or prevention. Phenothiazines are heterocyclic compounds with various pharmacological properties that are very adaptable for drug repurposing. In the present report, we analyzed the antiviral activity against DENV and the related Zika virus (ZIKV) of trifluoperazine (TFP), a phenothiazine derivative in clinical use as an antipsychotic and antiemetic agent. TFP exhibited dose-dependent inhibitory activity against the four DENV serotypes and ZIKV in monkey Vero cells at non-cytotoxic concentrations with 50% effective concentration values in the range 1.6-6.4 µM. A similar level of antiviral efficacy was exhibited by TFP against flavivirus infection in the human cell lines A549 and HepG2. Mechanistic studies, performed using time-dependent infectivity assays, real-time RT-PCR, Western blot, and immunofluorescence techniques, indicated that uncoating of the virus during penetration into the cell was the main target for TFP in infected cells, but the compound also exerted a minor effect on a late stage of the virus multiplication cycle. This study demonstrates that TFP, a pharmacologically active phenothiazine, is a selective inhibitor of DENV multiplication in cell culture. Our findings open perspectives for the repositioning of phenothiazines like TFP with a wide spectrum of antiviral efficacy as potential agents for the control of pathogenic flaviviruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue virus (DENV) causes endemic disease in tropical and subtropical regions of the world and represents an increasing public health threat for the human population. About 350 million annual infections transmitted by Aedes mosquitoes are estimated to occur [1], resulting in a wide spectrum of clinical manifestations that range from asymptomatic or mild febrile infections to severe forms associated with hemorrhage and shock with a lethal outcome [2]. DENV is a member of the family Flaviviridae, genus Flavivirus, and the virion is an enveloped particle with a positive-stranded RNA genome. The existence of four serotypes, DENV-1 to DENV-4, which co-circulate worldwide and give rise to sequential outbreaks, is one of the main determinants of the severity of the disease, since heterotypic reinfection is associated with progression to the more serious clinical presentations [3].
Neither of the two main strategies to combat viral infections, vaccines and antiviral drugs, is currently available for dengue. Among the many vaccine candidates that have been developed over the years, a tetravalent chimeric dengue vaccine based on the backbone of the attenuated yellow fever virus strain 17D was licensed a few years ago in highly endemic countries [4]. However, it has shown variable efficacy against the four DENV serotypes, and although it confers a high degree of protection in DENV-seropositive vaccine recipients, it is associated with an increased risk of severe dengue in seronegative individuals after vaccination [5].
Regarding antiviral development, numerous compounds targeting viral and host cell components have been found to be potent inhibitors of DENV infection in vitro [6]. Unfortunately, only a few candidates, such as chloroquine, balapiravir, celgosivir, prednisolone, and lovastatin have been evaluated in clinical trials, and the results obtained up to now have been disappointing [7,8,9,10,11,12]. Thus, the search for effective DENV antiviral agents for control of this harmful disease continues to be a major challenge.
The abovementioned clinical studies are representative of a strategy of using drugs that were approved previously for other therapeutic purposes as potential DENV drugs. In recent years, there has been renewed interest in the approach of drug repurposing, since it allows antivirals to be developed at lower cost. Information about safety is already available, and the compounds have been approved for clinical use in humans. In contrast, the development of new drugs is costly, potentially limiting their use in low-income American and Asiatic countries where dengue is endemic.
Phenothiazines are compounds with various pharmacological properties that are very adaptable for drug repurposing. They are heterocyclic compounds used in clinical settings to treat psychotic disorders due to their activities as modulators of diverse neurotransmitter receptors, such as dopamine, histamine H1, and serotonergic and cholinergic receptors [13]. Furthermore, phenothiazines have been reported to have calmodulin-binding ability, which consequently inhibits the associated calcium signal transduction activity [14]. In addition to their antipsychotic activity, phenothiazines exhibit a wide range of pharmacological activities, such as antiemetic, antihistaminic, antipruritic, analgesic, anti-inflammatory, anticancer, and anti-infective properties [13, 15].
The screening of libraries of clinically approved compounds has allowed the identification of three phenothiazines (prochlorperazine, trifluoperazine, and fluphenazine) as potent inhibitors of hepatitis C virus (HCV), another member of the family Flaviviridae [16, 17]. More recently, activity of prochlorperazine, a drug approved to treat migraine, nausea, and vomiting, was demonstrated to inhibit DENV infection by targeting viral binding and entry [18]. Trifluoperazine (TFP), other antipsychotic phenothiazine that is primarily used for schizophrenia treatment, was also found able to inhibit the multiplication of measles virus [19], Epstein-Barr virus [20], influenza virus [21], arenaviruses [22], and coronaviruses [23]. In the present study, the antiviral activity of TFP in DENV-infected cells was investigated, and the results suggest that this pharmacologically active compound may be considered a potential agent for dengue chemotherapy.
Materials and methods
Compound
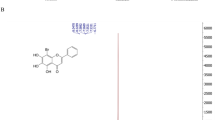
Trifluoperazine dihydrochloride (TFP) (Fig. 1a) was purchased from Sigma-Aldrich (USA). The stock solution was prepared in water at a concentration of 10 mM and sterilized by filtration.
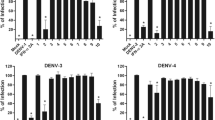
Antiviral activity of TFP against DENV and ZIKV in Vero cells. (a) Chemical structure of trifluoperazine dihydrochloride. (b-f) Cells were infected with DENV-1 (b), DENV-2 (c), DENV-3 (d), DENV-4 (e), or ZIKV (f), and after 24 h of infection in the presence of different concentrations of TFP, extracellular virus yields were determined by plaque assay. Data are mean values from three independent experiments ± standard deviation (SD)
Cells and viruses
Eagle's minimum essential medium (GIBCO, Thermo Fischer Scientific, USA) containing 5% newborn calf serum was used for propagation of the cell lines Vero (ATCC CCL 81), HepG2 (ATCC CRL-10741), and A549 (ATCC CL-185), and the serum concentration was decreased to 1.5% in maintenance medium (MM). For growth of C6/36 mosquito cells (ATCC CRL-1660) adapted to grow at 33°C, the culture medium consisted of L-15 medium (Leibovitz) (GIBCO) supplemented with 10% fetal bovine serum, 0.3% tryptose phosphate broth, 1% MEM non-essential amino acids, and 0.02% glutamine.
DENV-1 strain Hawaii, DENV-2 strain NGC, DENV-3 strain H87, DENV-4 strain 8124, and the clinical isolate INEVH116141 (provided by the Instituto Nacional de Enfermedades Virales Humanas, Pergamino, Argentina) of Zika virus (ZIKV) were grown in C6/36 cells. All viral stocks were titrated in Vero cells using a plaque assay.
Cytotoxicity assay
Cell cultures in 96-well microplates were treated with MM containing twofold serial dilutions of TFP. After 24 h of incubation at 37°C, cell viability was examined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma- Aldrich, USA) method as described previously [24]. The 50% cytotoxic concentration (CC50) was defined as the concentration of the compound that reduced absorbance by 50% in treated cells with respect to untreated control cells.
Virus yield reduction assay
Cells grown in 24-well microplates were infected with DENV or ZIKV at a multiplicity of infection (MOI) of 0.1 PFU/cell. After 1 h at 37°C for adsorption, the viral inoculum was removed and MM containing serial dilutions of TFP was added. A virus control without compound was also included. At 24 h postinfection (p.i.), virus yields in cell supernatants were measured by plaque assay in Vero cells. Assays were performed three times, each in quadruplicate. The effective concentration 50% (EC50), defined as the concentration of the compound able to reduce virus yield by 50% in treated cultures in comparison to untreated ones, was determined using a nonlinear regression of dose response inhibition with GraphPad Prism software.
Virucidal assay
Equivalent volumes of a suspension of DENV-2 (1 × 106 PFU/ml) and TFP solution (0-60 µM) were incubated at 37°C for 1 h. Then, the mixtures were chilled and diluted in MM, and the remaining infectivity was determined by titration.
Time-of-addition experiment
DENV-2 was allowed to adsorb to Vero cells (MOI, 0.1 PFU/cell) in MM with or without 30 µM TFP. After 1 h at 4°C, the inoculum was discarded, the cells were washed with cold phosphate-buffered saline (PBS), and MM with 30 µM TFP was added immediately (time, 1 h p.i.) or at 3, 5, or 7 h p.i. After 24 h of infection, extracellular virus yields were determined by plaque assay.
Virus adsorption, internalization, and uncoating assays
To measure virus adsorption, DENV-2-infected Vero cells (MOI, 1 PFU/cell) in the presence or absence of 30 µM TFP were incubated for 1 h at 4°C. After removing unadsorbed virus by washing with cold PBS, the cells were disrupted by freezing and thawing. Low-speed centrifugation was performed to remove cell debris, and the amount of infectious cell-bound virus was determined by plaque assay.
For virus internalization, DENV-2 was allowed to adsorb to Vero cells (MOI, 1 PFU/cell) for 1 h at 4°C in the absence of compound. Then, the cells were washed with PBS, and MM with or without 30 µM TFP was added. After incubation for 1 h at 37°C to allow virus internalization, the amount of internalized infectious virus was assessed using an infectious centre assay [24]. As a control, DENV-2 was allowed to adsorb at 4°C to other set of Vero cell cultures, and after further incubation at 37°C for 24 h in MM with or without 30 µM TFP, virus yields were determined by plaque assay.
To evaluate virion uncoating from endosomes, two assays were performed. First, DENV-2 (MOI, 10 PFU/cell) was allowed to adsorb to Vero cells grown on coverslips for 1 h at 4°C, after which the virus inoculum was removed and the cells were incubated at 37°C with prewarmed MM with or without 30 µM TFP to start internalization. After 0, 10, and 45 min, the cells were fixed with methanol for 10 min at -20°C and stained with a mouse monoclonal antibody against the DENV-2 C capsid protein (kindly provided by Dr. J. Aaskov, University of Queensland, Australia), followed by fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Sigma-Aldrich, USA). After staining of nuclei with Hoechst 33258, the cells were visualized under an Olympus fv-1000 confocal microscope with 600X amplification, and the number of bright spots was quantified in 200 randomly chosen cells.
Virion uncoating was also analyzed by monitoring the fate of cellular infectivity at early times p.i. DENV-2 was allowed to adsorb to Vero cells at 4°C for 1 h, the cells were washed with cold PBS, and virus internalization was initiated by incubation at 37°C with prewarmed MM with or without 30 µM TFP or 50 nM concanamycin A (Sigma-Aldrich, USA). After different times at 37°C, adsorbed but non-internalized virus was inactivated by treatment with citrate buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3) for 1 min. The cells were then washed with PBS and disrupted by freezing and thawing, and intracellular infectivity was titrated by plaque assay.
Detection of viral proteins by immunofluorescence and Western blot
For immunofluorescence, Vero cells seeded on coverslips were infected with DENV-2 and incubated at 37°C in MM with or without 30 µM TFP. At 24 h p.i., the cells were fixed with methanol, and sequential staining was performed. The primary antibody was a mouse monoclonal antibody against the DENV-2 E glycoprotein (Abcam, UK), and the secondary antibody was anti-mouse FITC-conjugated IgG. After staining of the cell nuclei with Hoechst 33258, the cells were examined using a fluorescence microscope (Olympus Corporation, Japan), and positive cells expressing the E glycoprotein were counted in 20 randomly chosen fields.
For Western blot assays, Vero cells were infected with DENV-2 and incubated for 24 h in MM with or without 30 µM TFP. Cell lysates were prepared in sample buffer (5% sodium dodecyl sulfate [SDS], 2% 2-mercaptoethanol, 10% glycerol, and 0.005% bromophenol blue in 0.0625 M Tris-HCl, pH 6.8) (Promega, USA), and proteins were separated by 10% SDS-polyacrylamide electrophoresis. After blotting onto a PVDF membrane (Millipore, USA) using a semi-dry system, the membranes were incubated overnight at 4°C with a rabbit antiserum against the NS5 protein of DENV-2 (kindly provided by Dr. A. Gamarnik, Instituto Leloir, Argentina). The membranes were then washed in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) containing 0.1% Tween 20 and incubated with horseradish-peroxidase-conjugated anti-rabbit IgG (Amersham, USA) for 1 h at room temperature. As a loading control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected using mouse anti-GAPDH (Abcam, UK) and peroxidase-conjugated anti-mouse IgG (Abcam, UK). Proteins bands were visualized by chemiluminescence (ECL, Amersham, USA).
Synthesis of viral RNA: real-time RT-PCR
Vero cells seeded in 6-well microplates were infected with DENV-2 (MOI: 0.1 PFU/cell) and incubated at 37°C with MM with or without 30 µM TFP. At 24 h p.i., total RNA was extracted from the cells using TRIzol Reagent (Invitrogen, USA) as instructed by the manufacturer, and a quantitative RT-PCR assay was performed employing TaqMan technology to amplify nucleotides 10,419 to 10,493 within the viral 3′UTR as described previously [24].
Statistical analysis
GraphPad Prism software was used to perform statistical analysis. For comparison of two groups of means, Student's unpaired t-test was used. Data normality was checked using the Shapiro-Wilks test, and homoscedasticity was tested using the Bartlett test. Data that did not meet the requirement of Gaussian distribution were analyzed using the non-parametric Kruskal-Wallis test. The kind of analysis performed is indicated in the figure legend. Data were always obtained from three independent experiments, with each one performed in quadruplicate. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001) is indicated in the figure.
Results
Cytotoxic and anti-flavivirus activity of TFP
Initially, the MTT method was employed to evaluate the cytotoxic effects of TFP on Vero cell viability after treatment with various concentrations of this compound. After determining the percentage of viable cells in TFP-treated and untreated Vero cell cultures, the CC50 was calculated to be 49.1 ± 6.0 µM (Table 1). A virus yield inhibition assay was then performed by infectivity titration of cell supernatants from DENV-infected cells treated with phenothiazine at concentrations lower than the CC50. The results shown in Fig. 1b-e demonstrated that the inhibitory action of TFP against DENV multiplication in Vero cells was dose-dependent. Furthermore, it should be noted that the compound was able to inhibit the propagation of all four DENV serotypes. Calculation of the EC50 value and selectivity index (SI), defined as the CC50/EC50 ratio, for each virus serotype allowed us to conclude that TFP exerted selective antiviral activity against DENV infection in Vero cells.
The antiviral effect of TFP was also evaluated against ZIKV, another relevant human-pathogenic flavivirus. As seen in Fig. 1F, ZIKV was also significantly inhibited by TFP, with an SI of 30.7 (Table 1).
The human cell lines A549 and HepG2 were also tested to examine the influence of the host cell on the inhibition of flavivirus infection by TFP. For that purpose, the susceptibility of DENV-2 and ZIKV infection to the drug was evaluated using a virus yield antiviral assay. Both flaviviruses were found to be sensitive to the antiviral action of TFP in A549 and HepG2 cells (Fig. 2a-d), with the level of cytotoxicity in the human cell lines similar to that observed in monkey Vero cells (Table 1).
Antiviral activity of TFP against DENV-2 and ZIKV in human cells. A549 (black bars) and HepG2 (white bars) cells were infected with DENV-2 (a-b) or ZIKV (c-d), and after 24 h of infection in the presence of different concentrations of TFP, extracellular virus yields were determined by plaque assay. Data are mean values from three independent experiments ± SD.
Lack of virucidal activity of TFP
The study of the mode of action of TFP on DENV infection was started by evaluating the possibility of a direct interaction of the drug with the virus particle leading to its inactivation. A virus suspension was incubated with different concentrations of the compound for 1 h at 37°C, followed by sample dilution and titration of the remaining infectivity. It was concluded that TFP had no observable virucidal effect on DENV-2 (Fig. 3).
Mode of action: effect of TFP on DENV entry
As TFP was similarly effective against the four DENV serotypes, DENV-2 in Vero cells was chosen as a representative model system to further study the mode of action of this compound against DENV infection. Studies were performed at a TFP concentration of 30 µM, about fivefold higher than the EC50, to achieve a strong inhibitory effect in all of the assays. In an attempt to localize the antiviral target of TFP in the viral cycle of DENV-2, the impact of the time of addition of the compound on its inhibitory effect was evaluated. For that purpose, TFP was added to cell cultures simultaneously with virus or at various times after infection, and virus production was measured after 24 h of infection. The results of this time-course study, shown in Fig. 4a, indicate that the strongest inhibitory effect was observed when the compound was added to cells together with the virus (time 0) or immediately after adsorption at 1 h p.i. Under both experimental conditions, the amount of virus released from infected cells was reduced by more than 90%. However, addition of TFP at later times after infection produced a lower but consistent decrease in virus production, attaining around 50% inhibition.
Mode of antiviral action of TFP: effect on virus entry. (a) Vero cells were infected with DENV-2, and 30 µM TFP was added simultaneously with virus (time 0) or at the indicated times after infection. Extracellular virus yields were determined at 24 h p.i. in all cell cultures. Statistical significance was evaluated using the nonparametric Kruskal-Wallis test. (b) DENV-2 was allowed to adsorb to Vero cells at 4°C for 1 h in MM with or without 30 µM TFP. Then, the amount of cell-bound infectious virus was determined by plaque assay (Adsorption). In other Vero cell cultures, DENV-2 was allowed to adsorb at 4°C for 1 h. After further incubation at 37°C for 1 h in MM with or without 30 µM TFP, cell monolayers were treated with proteinase K, and internalized virus was detected using an infectious center assay (Internalization). Finally, DENV-2 was allowed to adsorb at 4°C to another set of cultures, and after further incubation at 37°C for 24 h in MM with or without 30 µM TFP, virus yields in the cell supernatants were determined by plaque assay (Always). Statistical significance for each treatment was evaluated using Student's t-test. (c) DENV-2 was allowed to adsorb to Vero cells at 4°C in the absence (VC) or presence (TFP) of 30 µM TFP, and cultures were then shifted to 37°C. At 0, 10, and 45 min, cells were fixed and processed for immunofluorescence to detect the C protein. Cell nuclei were stained with Hoechst 33258. (d) Vero cells were infected with DENV-2 for 1 h at 4°C and then incubated with MM (VC) or MM containing 30 µM TFP (TFP) or 50 nM concanamycin A (ConA). At different times post-adsorption, non-internalized virus was inactivated, cells were disrupted, and intracellular infectivity was determined. Statistical significance was evaluated using the nonparametric Kruskal-Wallis test. In a, b, and d, each value represents the mean of three independent experiments ± SD. NS, not significant; *, p < 0.05; **,p < 0.01; ***, p < 0.001; ****, p < 0.0001
Next, the apparent antiviral action of TFP on the early steps of DENV infection was evaluated further. For this purpose, the quantity of adsorbed or internalized virus was determined when TFP was present exclusively during the virus adsorption or virus internalization step. When DENV-2 was allowed to adsorb to Vero cells at 4°C in the presence of 30 µM TFP, cell-bound infectivity remained unaltered (Fig. 4b), but a different effect was observed if the compound was absent during virus adsorption at 4°C but present immediately afterwards when infected cultures were then incubated at 37°C for 1 h. In the presence of TFP, the amount of internalized virus, quantified using an infectious center assay, was significantly reduced; however, a higher inhibition rate was observed when the compound was present throughout the entire infection cycle (Fig. 4b). These results confirm the data from the time-of-addition assay: treatment with TFP mainly affected the early step of virus entry into the cells, but a later step might also be affected by this compound.
The next step in the process of DENV entry into the cell is virion uncoating, when fusion of the viral envelope with the endosomal membrane takes place and the nucleocapsid is released into the cytoplasm, leading to the consequent loss of capsid from the genome to start macromolecular synthesis. In order to evaluate if uncoating of DENV-2 is affected by TFP, the intracellular presence of capsid protein was monitored by immunofluorescence staining of infected cells during the period of internalization at 37°C. It has been demonstrated previously that the C protein can be detected in the cytoplasm of DENV-2-infected cells a few minutes after infection and then progressively becomes undetectable [25]. Accordingly, when DENV-2 infection was performed in the absence of TFP, a bright dotted fluorescent pattern of C protein was observed at 10 min p.i., whereas fluorescence was strongly decreased at 45 min p.i. (Fig. 4c), indicating the loss of C protein, initially from endosomes, and later from the cytoplasm. On the other hand, when infection occurred in the presence of TFP, the dotted C protein fluorescence was maintained in the cytoplasm during internalization (Fig. 4c), suggesting that uncoating and release of DENV-2 from the endosomes was inhibited by TFP. Quantification of the fluorescence corresponding to C protein in control infected cells showed a reduction of 55% in the number of fluorescent particles inside the cell at 45 min p.i., whereas in infected cells treated with TFP, the level of the fluorescent signal was maintained in the same period of time with a reduction of only 6%.
The effect on virion uncoating was also studied by recording the fate of infectious virus inside the cell during the early phase of infection. To this end, DENV-2 was allowed to adsorb to Vero cells at 4°C, after which internalization was allowed in the presence or absence of TFP for different times p.i. at 37°C and cell-associated infectivity was titrated. As expected, a significant reduction of the intracellular infectivity as a consequence of virion uncoating was detected in control infected cells, but in TFP-treated cells, the presence of infectious virus inside the cell was not significantly affected (Fig. 4d). Concanamycin A, a compound that is known to allow endocytosis but to be able to block low-pH-dependent membrane fusion, preventing release from endosomes of nucleocapsids [24], was also tested in this assay as a positive control. As seen in Fig. 4d, the number of infectious intracellular DENV-2 virions in concanamycin-A-treated Vero cells was similar to that observed in TFP-treated cells, confirming that viral uncoating seems to be the step in the early stage of infection that is affected by TFP.
Effect of TFP on viral RNA synthesis and protein expression
To further explore the outcome of DENV infection in the presence of TFP, viral macromolecular synthesis in treated infected cells was next investigated. A significant inhibition of viral RNA synthesis was detected by quantitative RT-PCR in infected Vero cells after treatment with TFP (Fig. 5a), as well as in the level of DENV-2 E protein expression detected by indirect immunofluorescence (Fig. 5b). A reduction of more than 85% was observed in both the number of cell-associated genome copies and the number of cells expressing the E protein in DENV-2-infected cultures in the presence of 30 µM TFP in comparison with untreated infected cells. The reduction in viral protein synthesis was also confirmed by examining the expression of the DENV-2 non-structural protein NS5 by Western blot. As seen in Fig. 5c, the amount of NS5 was much lower in TFP-treated infected cells than in control infected cells. Altogether, these suggest that TFP inhibits viral uncoating, macromolecular synthesis, and virus production, thereby affecting the spread of infection.
Effect of TFP on DENV-2 RNA and protein expression. (a) DENV-2-infected Vero cells were treated (TFP) or not treated (VC) with 30 µM TFP. At 24 h p.i., total RNA was extracted from the cells to determine the amount of viral RNA by qRT-PCR. Results are expressed as viral RNA copies, and data are mean values from three independent experiments ± SD. Statistical significance was evaluated using an unpaired t-test (*, p < 0.05). (b) Vero cells treated (TFP) or not treated (VC) with TFP as in panel a were fixed and processed for immunofluorescence to detect the E glycoprotein. Cell nuclei were stained with Hoechst 33258. Magnification, 600X. (c) DENV-2-infected Vero cells were treated (TFP) or not treated (VC) with 30 µM TFP, and at 24 h p.i. the expression of NS5 protein was analyzed by Western blot. GAPDH was used as a loading control. CC, control uninfected cells
Discussion
The studies reported here demonstrate that the phenothiazine derivative TFP is an active inhibitor of the in vitro multiplication of flaviviruses, exhibiting effective antiviral activity against the four serotypes of DENV and the related ZIKV, an arbovirus found in co-circulation with DENV in endemic areas that also exhibits antigenic cross-reactivity [26,27,28]. Since this makes differential diagnosis more difficult, the potential availability of an antiviral drug that is effective against both DENV and ZIKV that can be rapidly administered to patients might be especially useful. The level of viral inhibition varied according to the virus and host cell tested, but in all cases, the compound affected virus infection at noncytotoxic concentrations, with EC50 values between 1.6 and 6.4 µM and the SI in the range of 5.0-30.7 (Table 1). These selectivity values are similar or even higher than those reported for TFP in other virus-cell systems [16, 17, 22, 23, 29]. No inactivating activity of TFP against DENV was detected when a suspension of virus particles was pre-treated with the drug before cell infection, indicating that the observed viral inhibition was due to a blockade of virus multiplication during the infectious process and not to interaction of the drug with the virion.
From the results of the time-of-addition studies, it can be deduced that the early steps leading to virus entry are the main targets of TFP during DENV infection, since the greatest reduction in virus yield was achieved when the phenothiazine was added during the first hour of infection (Fig. 4a). However, a minor antiviral effect was observed when the compound was added at a later time point, indicating a possible effect of TFP on a late stage of the virus multiplication cycle.
When the effect of TFP on the early steps of DENV-2 infection was analyzed, the C protein, as well as infectious virus, remained detectable inside the cell for a longer period of time than in untreated infected cells, in which a rapid loss of capsid protein and intracellular infectivity was observed (Fig. 4c and d). Furthermore, the initial virus binding to the cell was not disturbed (Fig. 4b). These data suggest that virions entered the cell but that the fusion of the viral envelope with the endosomal membrane, leading to egress from the endosome and uncoating of the nucleocapsid, is hindered by TFP. Studies with HCV have also demonstrated that phenothiazines affect endocytic cellular processes required for virus penetration and that this effect is similar to that observed after treatment with lysosomotropic compounds that neutralize endosomal pH [16, 17]. Other studies have shown that the blockade of HCV infectious entry by phenothiazines occurs at the step of fusion with the host cell membrane by causing alterations in the lipid domains of the target membrane [29, 30]. It is possible that the same mechanism of membrane perturbation is responsible for the observed anti-DENV activity of TFP, not only at the early stage but also at later stages of the virus multiplication cycle, as shown in the time-of-addition experiment in Fig. 4a. Since all of the intracellular steps of flavivirus multiplication occur in association with cellular membranes, the lower degree of inhibition of DENV production observed when TFP is added after virus penetration may be due to interference with other stages of the viral cycle, such as macromolecular synthesis, virion assembly, or budding. In fact, we detected a significant inhibition of DENV-2 RNA replication in the presence of TFP, in addition to the effect on virus entry (Fig. 5a). Consistent with this, a minor effect of TFP on HCV RNA replication, with a 40-60% reduction in RNA synthesis, has been reported, whereas virus entry was inhibited by more than 80%, suggesting an inhibitory activity of TFP on multiple steps of the HCV replicative cycle [16]. Partial interference with the final stages of virion assembly and budding might also be possible, since TFP has been shown to be an inhibitor of the morphogenesis of influenza virus in MDCK cells [21] and to block the budding of measles virus in HeLa cells [19].
It is known that TFP, like other phenothiazine derivatives, affects diverse events in the host cell, but with two main classical roles: antagonism of dopamine receptors in neural signaling networks and binding to calmodulin, interfering with the modulation of several Ca2+-dependent enzymes or pathways. Both cellular factors appear to be involved in DENV infection, although their specific role in the DENV replicative cycle is not clearly known: calmodulin antagonists such as sulfonamide derivatives have been reported to be inhibitors of DENV infection, reducing viral translation/replication and viral yield [31, 32], whereas the positive association of dopamine 2 receptor (D2R) with DENV has been shown using D2R antagonists [18, 33, 34]. The precise cellular target of TFP responsible for the blockade of DENV infection remains to be identified, but the observed variety of effects of this class of compounds on cells makes it more likely to affect different events in the viral cycle, as observed here.
In conclusion, TFP exhibited antiviral activity against the four DENV serotypes and ZIKV at noncytotoxic concentrations, showing that this drug may have broad antiviral potential against different human-pathogenic members of the family Flaviviridae. Since both DENV and ZIKV co-circulate in endemic areas of America and Asia, as well as other arboviruses such as chikungunya virus (CHIKV), the prospects of the repurposing phenothiazines such as TFP with a wide spectrum of antiviral efficacy are promising. Although it is well known that the use of TFP as an antipsychotic drug can cause undesirable side effects in patients after prolonged periods of administration, since dengue is an acute disease, the duration of chemotherapy is limited in time, reducing the likelihood of unwanted complications. Prophylactic or therapeutic treatment with TFP in at-risk persons or patients with initial signs before a precise diagnosis may improve the chances of reducing virus transmission and be helpful for the control of epidemic outbreaks.
References
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen SI, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504-507.https://doi.org/10.1038/nature12060
Guzmán MG, Harris E (2015) Dengue. Lancet 385:453–465. https://doi.org/10.1016/S0140-6736(14)60572-9
Guzmán MG, Alvarez M, Halstead SB (2013) Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol158:1445-1459.https://doi.org/10.1007/s00705-013-1645-3
Guy B, Noriega F, Ochiai RL, L’azou M, Delore V, Skipetrova A, Verdier F, Coudeville L, Savarino S, Jackson N (2017) A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev Vaccines 7:1–13. https://doi.org/10.1080/14760584.2017.1335201
Thomas ST, Yoon I-K (2019) A review of Dengvaxia®: development to deployment. Hum Vaccin Immunother 15:2295–2314. https://doi.org/10.1080/21645515.2019.1658503
Troost B, Smit JM (2020) Recent advances in antiviral drug development towards dengue virus. CurrOpinVirol43:1-13.https://doi.org/10.1016/j.coviro.2020.07.009
Borges MC, Castro LA, Fonseca BA (2013)Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz 108:596-599.https://doi.org/10.1590/S0074-02762013000500010
Nguyen NM, Tran CNB, Phung LK, Duong KTH, HuynhHA, Farrar J, Nguyen QTH, Tran HT, Nguyen CVV, Merson L, Hoang LT, Hibberd ML, Aw PPK, Wilm A, Nagarajan N, Nguyen DT, Pham MP, Nguyen TT, Javanbakht H, Klumpp K, Hammond J, Petric R, Wolbers M, Nguyen CT, Simmons CP (2013) A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 207:1442-1450.https://doi.org/10.1093/infdis/jis470
Sung C, Wei Y, Watanabe S, Lee HS, Khoo YM, Fan L, Rathore APS, Chan KW-K, Choy MM, Kamaraj US, Sessions OM, Aw P, de Sessions PF, Lee B, Connolly JE, Hibberd ML, Vijaykrishna D, Wijaya L, Ooi EE, Low JG-H, Vasudevan SG (2016) Extended evaluation of virological, immunological and pharmacokinetic endpoints of CELADEN: a randomized, placebo-controlled trial of celgosivir in dengue fever patients. PLoSNeglTropDis 10:e0004851. https://doi.org/10.1371/journal.pntd.0004851
Tam DTH, Ngoc TV, Tien NTH, Kieu NTT, Thuy TTT, Thanh LTC, Tam CT, Truong NT, Dung NT, Qui PT, Hien TT, Farrar J, Simmons CP, Wolbers M, Wills BA (2012) Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 55:1216-1224.https://doi.org/10.1093/cid/cis655
Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP (2010) A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoSNeglTropDis 4:e785. https://doi.org/10.1371/journal.pntd.0000785
Whitehorn J, Nguyen CVN, Khanh LP, Kien DTH, Quyen NTH, Tran NTT, Hang NT, Truong NT, Tai LTH, Huong NTC, Nhon VT, Tram TV, Farrar J, Wolbers M, Simmons CP, Wills B (2016) Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 62:468–476. https://doi.org/10.1093/cid/civ949
Varga B, Csonka A, Csonka A, Molnár J, Amaral L, Spengler G (2017) Possible biological and clinical applications of phenothiazines. Anticancer Res 37:5983–5993. https://doi.org/10.21873/anticanres.12045
Weiss B, Prozialeck WC, Wallace TL (1982) Interaction of drugs with calmodulin. Biochemical, pharmacological and clinical implications. BiochemPharmacol31:2217-2226.https://doi.org/10.1016/0006-2952(82)90104-6
Pluta K, Morak-Mlodawska B, Jelen M (2011) Recent progress in biological activities of synthesized phenotiazines. Eur J Med Chem 46:3179-3189.https://doi.org/10.1016/j.ejmech.2011.05.013
Chockalingam K, Simeon RL, Rice CM, Chen Z (2010) A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc Natl AcadSci USA107:3764-3769. https://doi.org/10.1073/pnas.0915117107
Gastaminza P, Whitten-Bauer C, Chisari FV (2010) Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. ProcNatlAcadSci USA 107:291-296. www.pnas.org/cgi/doi/https://doi.org/10.1073/pnas.0912966107
Simanjuntak Y, Liang JJ, Lee YL, Lin YL (2015) Repurposing of prochlorperazine for use against dengue virus infection. J Infect Dis 211:394-404.https://doi.org/10.1093/infdis/jiu377
Bohn W, Rutter G, Hohenberg H, Mannweiler K (1983) Inhibition of measles virus budding by phenotiazines. Virology 130:40-55.https://doi.org/10.1016/0042-6822(83)90116-2
Nemerow GR, Cooper NR (1984) Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus is blocked by calmodulin antagonists. Proc Natl AcadSci USA 81:4955-4959.https://doi.org/10.1073/pnas.81.15.4955
Ochiai H, Kurokawa M, Niwayama S (1991) Influence of trifluoperazine on the late stage of influenza virus infection in MDCK cells. Antiviral Res15:149-160.https://doi.org/10.1016/0166-3542(91)90032-M
Candurra NA, Maskin L, Damonte EB (1996) Inhibition of arenavirus multiplication in vitro by phenotiazines. Antiviral Res 31:149-158.https://doi.org/10.1016/0166-3542(96)06956-2
Xiao X, Wang C, Chang D, Wang Y, Dong X, Jiao T, Zhao Z, Ren L, Dela Cruz CS, Sharma L, Lei X, Wang J (2020) Identification of potent and safe antiviral therapeutic candidates against SARS-CoV-2. Front Immunol 11:586572.https://doi.org/10.3389/fimmu.2020.586572
Talarico LB, Damonte EB (2007) Interference in dengue virus adsorption and uncoating by carrageenans. Virology 363:473485. https://doi.org/10.1016/j.virol.2007.01.043
Acosta EG, Castilla V, Damonte EB (2012) Differential Requirements in Endocytic Trafficking for Penetration of Dengue Virus. PLoS ONE 7(9):e44835. https://doi.org/10.1371/journal.pone.0044835
Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR (2016) Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17:1102–1108. https://doi.org/10.1038/ni.3515
Rico-Mendoza A, Porras-Ramírez A, Chang A, Encinales L, Lynch R (2019) Co- circulation of dengue, chikungunya, and Zika viruses in Colombia from 2008 to 2018. Rev PanamSaludPublica43:e49.https://doi.org/10.26633/RPSP.2019.49
Rothan HA, Bidokhti MRM, Byrareddyb SN (2018) Current concerns and perspectives on Zika virus co-infection with Arboviruses and HIV. J Autoimmun89:11-20.https://doi.org/10.1016/j.jaut.2018.01.002
Chamoun-Emanuelli AM, Pecheur EI, Simeon RL, Huang D, Cremer PS, Chen Z (2013) Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes. Antimicrob Agents Chemother 57:2571–2581. https://doi.org/10.1128/AAC.02593-12
Perin PM, Haid S, Brown RJ, Doerrbecker J, Schulze K, Zeilinger C, von Schaewen M, Heller B, Vercauteren K, Luxenburger E, Baktash YM, Vondran FW, Speerstra S, Awadh A, Mukhtarov F, Schang LM, Kirschning A, Müller R, Guzman CA, Kaderali L, Randall G, Meuleman P, Ploss A, Pietschmann T (2016)Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1. Hepatology 63:49-62.https://doi.org/10.1002/hep.28111
Bautista-Carvajal P, Soto-Acosta R, Angel-Ambrocio AH, Cervantes-Salazar M, Loranca-Vega CI, Herrera-Martinez M, del Angel RM (2017) The calmodulin antagonist W-7 (N-(6-aminohexyl)-5-chloro-1-naphthalaenesulfonamide hydrochloride) inhibits DENV infection in Huh-7 cells. Virology 501:188-198.https://doi.org/10.1016/j.virol.2016.12.004
Chen W-C, Simanjuntak Y, Chu L-W, Ping Y-H, Lee Y-L, Lin, Y-L, Li W-S (2020) Benzene sulfonamide derivatives as calcium/calmodulin-dependent proteína kinase inhibitors and antiviral agents against dengue and Zika virus infections. J Med Chem 63:1313-1327.https://doi.org/10.1021/acs.jmedchem.9b01779
Ho M-R, Tsai T-T, Chen C-L, Jhan M-K, Tsai C-C, Lee Y-C, Chen C-H, Lin C-F (2017) Blockade of dengue virus infection and viral cytotoxicity in neuronal cells in vitro and in vivo by targeting endocytic pathways. Sci Rep 7:6910.https://doi.org/10.1038/s41598-017-07023-z
Shen T-J, Hanh VT, Nguyen TQ, Jhan M-K, Ho M-R, Lin C-F (2021) Repurposing the antiemetic metoclopramide as an antiviral against dengue virus infection in neuronal cells. Front Cell Infect Microbiol 10:606743. https://doi.org/10.3389/fcimb.2020.606743
Acknowledgements
This work was supported by grants from Universidad de Buenos Aires (20020170100363BA) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015 3080), Argentina. EBD is member of Research Career from CONICET.
Author information
Authors and Affiliations
Contributions
LEP: performed the experiments and analyzed the data. VC and EBD: designed the study, analyzed the data, and wrote the manuscript. All authors read the manuscript and approved its submission to Archives of Virology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of data and materials
All data generated and analysed during this study are available in this article.
Additional information
Handling Editor: Zhenhai Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piccini, L.E., Castilla, V. & Damonte, E.B. Inhibition of dengue virus infection by trifluoperazine. Arch Virol 167, 2203–2212 (2022). https://doi.org/10.1007/s00705-022-05555-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05555-y