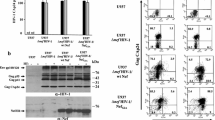
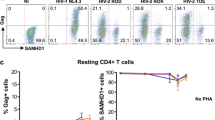
Abstract
Intact HIV-1 and exosomes can be internalized by dendritic cells (DCs) through a common pathway leading to their transmission to CD4+ T lymphocytes by means of mechanisms defined as trans-infection and trans-dissemination, respectively. We previously reported that exosomes from HIV-1-infected cells activate both uninfected quiescent CD4+ T lymphocytes, which become permissive to HIV-1, and latently infected cells, with release of HIV-1 particles. However, nothing is known about the effects of trans-dissemination of exosomes produced by HIV-1-infected cells on uninfected or latently HIV-1-infected CD4+ T lymphocytes. Here, we report that trans-dissemination of exosomes from HIV-1-infected cells induces cell activation in resting CD4+ T lymphocytes, which appears stronger with mature than immature DCs. Using purified preparations of both HIV-1 and exosomes, we observed that mDC-mediated trans-dissemination of exosomes from HIV-1-infected cells to resting CD4+ T lymphocytes induces efficient trans-infection and HIV-1 expression in target cells. Most relevant, when both mDCs and CD4+ T lymphocytes were isolated from combination anti-retroviral therapy (ART)-treated HIV-1-infected patients, trans-dissemination of exosomes from HIV-1-infected cells led to HIV-1 reactivation from the viral reservoir. In sum, our data suggest a role of exosome trans-dissemination in both HIV-1 spread in the infected host and reactivation of the HIV-1 reservoir.









Similar content being viewed by others
References
Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM (1992) Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytophathic infection to CD4+ T cells. Science 257:383–386
Geijtenbeek TBH, Kwon DS, Torensma R et al (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597
McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ (2003) Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297
Piguet V, Sattentau Q (2004) Dangerous liaisons at the virological synapse. J Clin Investig 114:605–610
Laguette N, Sobhian B, Casartelli N et al (2011) SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657
Ryoo J, Choi J, Oh C et al (2014) The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 20:936–941
Garcia E, Pion M, Pelchen-Matthews A et al (2005) HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488–501
Yu HJ, Reuter MA, McDonald D (2008) HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PloS Pathog 4:e1000134
Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S (2012) Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J Infect Dis 205:1248–1257
Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JHN, Kapsenberg ML, Berkhout B (2002) Differential transmission of human immunodeficiency virus type I by distinct subsets of effector dendritic cells. J Virol 76:7812–7821
Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu XW, Reinhard BM, Gummuluru S (2013) Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on siglec-1/CD169. PloS Pathog 9(4):e1003291
Izquierdo-Useros N, Lorizate M, Puertas MC et al (2012) Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PloS Biol 10(12):e1001448
Izquierdo-Useros N, Lorizate M, Contreras FX et al (2012) Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol 10(4):e1001315
Puryear WB, Yu XW, Ramirez NP, Reinhard BM, Gummuluru S (2012) HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci USA 109:7475–7480
Schorey JS, Cheng Y, Singh PP, Smith VL (2015) Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep 16:24–43
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteom 73:1907–1920
Skog J, Wurdinger T, van Rijn S et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Hubert A, Barbeau B, Subra C, Bissonnette L, Gilbert C (2015) Role and future applications of extracellular vesicles in HIV-1 pathogenesis. Future Virol 10:357–370
Madison MN, Okeoma CM (2015) Exosomes: implications in HIV-1 pathogenesis. Viruses 7:4093–4118
Teow SY, Nordin AC, Khoo ASB (2016) Exosomes in human immunodeficiency virus type I pathogenesis: threat or opportunity? Adv Virol. doi:10.1155/2016/9852494
Lee JH, Wittki S, Brau T et al (2013) HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol Cell 49:668–679
Gooz M (2010) ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol 45:146–169
Arenaccio C, Chiozzini C, Columba-Cabezas S et al (2014) Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4(+) T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol 88:11529–11539
Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Federico M (2014) Cell activation and HIV-1 replication in unstimulated CD4+ T lymphocytes ingesting exosomes from cells expressing defective HIV-1. Retrovirology 11:46. doi:10.1186/1742-4690-11-46
Arenaccio C, Anticoli S, Manfredi F, Chiozzini C, Olivetta E, Federico M (2015) Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 12:87. doi:10.1186/s12977-015-0216-y
Izquierdo-Useros N, Naranjo-Gomez M, Archer J et al (2009) Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113:2732–2741
Adachi A, Gendelman HE, Koenig S et al (1986) Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–2891
Federico M, Titti F, Butto S et al (1989) Biologic and molecular characterization of producer and non-producer clones from HUT-78 cells infected with a patient HIV isolate. AIDS Res Hum Retrovir 5:385–396
Sparacio S, Pfeiffer T, Schaal H, Bosch V (2001) Generation of a flexible cell line with regulatable, high-level expression of HIV Gag/Pol particles capable of packaging HIV-derived vectors. Mol Ther 3:602–612
Federico M, Percario Z, Olivetta E et al (2001) HIV-1 Nef activates STAT1 in human monocytes/macrophages through the release of soluble factors. Blood 8:2752–2761
Dettenhofer M, Yu XF (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of vif in virions. J Virol 73:1460–1467
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 Chapter 3, Unit 3.22
Wu L, KewalRamani VN (2006) Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6:859–868
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ (2010) Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190:1079–1091
Kogure T, Lin WL, Yan IK, Braconi C, Patel T (2011) Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 54:1237–1248
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452
Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T (2012) Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem 287:1397–1405
Trajkovic K, Hsu C, Chiantia S et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Yuyama K, Sun H, Mitsutake S, Igarashi Y (2012) Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem 287:10977–10989
Liu YJ, Kanzler H, Soumeli V, Gilliet M (2001) Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol 2:585–589
Sanchez G, Xu XY, Chermann JC, Hirsch I (1997) Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J Virol 71:2233–2240
Fourati S, Lambert-Niclot S, Soulie C et al (2012) HIV-1 genome is often defective in PBMCs and rectal tissues after long-term HAART as a result of APOBEC3 editing and correlates with the size of reservoirs. J Antimicrob Chemother 67:2323–2326
Janini M, Rogers M, Birx DR, McCutchan F (2001) Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4(+) T cells. J Virol 75:7973–7986
Cantin R, Diou J, Belanger D, Tremblay AM, Gilbert C (2008) Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J Immunol Methods 338:21–30
Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M (2000) Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha 4 beta 1. Eur J Biochem 267:583–590
Wiley RD, Gummuluru S (2006) Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA 103:738–743
Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P (2012) Mechanisms of dendritic cell trafficking across the blood–brain barrier. J Neuroimmune Pharmacol 7:74–94
Cohn LB, Silva IT, Oliveira TY et al (2015) HIV-1 integration landscape during latent and active infection. Cell 160:420–432
Barton K, Winckelmann A, Palmer S (2016) HIV-1 reservoirs during suppressive therapy. Trends Microbiol 24:345–355
Palmer S, Maldarelli F, Wiegand A et al (2001) Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA 105:3879–3884
Acknowledgements
We thank Massimo Sanchez, Istituto Superiore di Sanità, for the support in FACS analysis, and Pietro Arciero and Stefania de Menna, Istituto Superiore di Sanità, for their excellent technical support. Both AZT and anti-Env gp120 mAb were obtained from the NIH AIDS Research and Reference Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was supported by a Grant from “Ricerca Finalizzata” project RF-2010-2308334 from the Ministry of Health, Italy. CC, CA, EO, SA, FM, FF, G d’E, IS, MA, and MF declare that they have no conflict of interest. This article does not contain studies with animals. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chiozzini, C., Arenaccio, C., Olivetta, E. et al. Trans-dissemination of exosomes from HIV-1-infected cells fosters both HIV-1 trans-infection in resting CD4+ T lymphocytes and reactivation of the HIV-1 reservoir. Arch Virol 162, 2565–2577 (2017). https://doi.org/10.1007/s00705-017-3391-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3391-4