Abstract
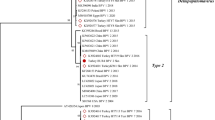
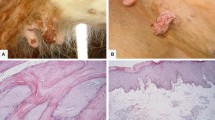
Papillomaviruses (PVs) are epitheliotropic viruses that cause benign proliferative lesions in the skin (warts or papillomas) and mucous membranes of their natural hosts. In bovines specifically, 13 types of Bovine papillomaviruses (BPVs) are currently described in the literature, although the actual number may be greater than 20. BPV types are classified into four genera based on homology within the genomic regions of the L1 ORF, the most conserved sequence. This study conducted molecular typing of BPV in dairy cows with different papillomatosis cases and investigated the presence of co-infections across distinct BPV types in the same sample. After carrying out PCR using degenerate primers and type specific primers, 35 BPV suspected samples were detected as positive for BPV and these samples were used for typing using sequence analysis/PCR with type-specific primers. This analysis identified BPV-1, -2, -3, -4, -6, -7, -9 and -10, new putative types (BPV/BR/UEL6-like viruses) and the previously described putative type viruses (BAPV-6) in the 35 BPV-positive samples. In addition, co-infections across different BPV types were widely detected in the BPV-positive samples. This study shows that PCR assays using degenerate primers to amplify partial fragments of the L1 gene followed by sequencing is useful for genotyping BPV. However, results need confirmation using type-specific primers in order to consider co-infections. In addition, this study identified a new putative type (in the same cluster as BPV/BR/UEL6-like viruses) and the previously described putative type viruses (BAPV-6) in teat papillomatosis of Turkish dairy cows. The study shows that it is essential to identify BPV types and their prevalence/distribution, and also to determine the clinical consequences of infection for the development of prophylactic and/or therapeutic procedures.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 17:3389–3402
Antonsson A, Hansson BG (2002) Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. J Virol 76:12537–12542
Ataseven VS, Kanat Ö, Ergün Y (2016) Molecular identification of bovine papillomaviruses in dairy and beef cattle: First description of Xi- and Epsilonpapillomaviruses in Turkey. Turk J Vet Anim Sci. doi:10.3906/vet-1512-1564
Bastawecy IM (2012) Isolation of bovine herpesvirus-2 (BHV-2) from a case of pseudo-lumpy skin disease in Egypt. J Am Sci 8:2
Batista MV, Silva MA, Pontes NE, Reis MC, Corteggio A, Castro RS, Borzacchiello G, Balbino VQ, Freitas AC (2013) Molecular epidemiology of bovine papillomatosis and the identification of a putative new virus type in Brazilian cattle. Vet J 197:368–373
Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM (2010) Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79
Bocaneti F, Altamura G, Corteggio A, Velescu E, Roperto F, Borzacchiello G (2016) Bovine papillomavirus: New insights into an old disease. Transbound Emerg Dis 63:14–23
Borzacchiello G, Roperto F (2008) Bovine papillomaviruses, papillomas and cancer in cattle. Vet Res 39:45
Campo MS (1987) Papillomas and cancer in cattle. Cancer Surv 6:39–54
Campo MS (2006) Bovine Papillomavirus: old system, new lessons?. Caister Academic Press, England (Chapter 23)
Carvalho CCR, Batista MVA, Silva MAR, Balbino VQ, Freitas AC (2012) Detection of bovine papillomavirus types, co-infection and new BPV11 subtype in cattle. Transbound Emerg Dis 59:441–447
Claus MP, Lunardi M, Alfieri AF (2008) Identification of unreported putative new bovine papillomavirus types in Brazilian cattle herds. Vet Microbiol 132:396–401
Claus MP, Lunardi M, Alfieri AF (2009) Identification of the recently described new type of bovine papillomavirus (BPV-8) in a Brazilian beef cattle herd. Pesqui Vet Bras 29:25–28
Claus MP, Vivian D, Lunardi M (2007) Phylogenetic analysis of bovine papillomavirus associated with skin warts in cattle herds from the state of Parana. Pesqui Vet Bras 27:314–318
Corteggio A, Altamura G, Roperto F, Borzacchiello G (2013) Bovine papillomavirus E5 and E7 oncoproteins in naturally occurring tumors: are two better than one? Infect Agents Cancer 8:1
de Villiers EM, Fauquet C, Broker TR, Bernard HU, Hausen H (2004) Classification of papillomaviruses. Virology 1:17–27
Egyed J, Ballagi-Pordany A, Bartha A, Belak S (1996) Studies of in vivo distribution of bovine herpesvirus type 4 in the natural host. J Clin Microbiol 34:1091–1095
Forslund O, Antonsson A, Nordin P (1999) A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol 80:2437–2443
Goltz M, Broll H, Mankertz A, Weıgelt W, Ludwıg H, Buhk HJ, Borchers K (1994) Glycoprotein B of bovine herpesvirus type 4: Its phylogenetic relationship to gB equivalents of the herpesviruses. Virus Genes 9:53–59
Grindatto A, Ferraro G, Varello K, Crescio M, Miceli I, Bozzetta E, Goria M, Nappi R (2015) Molecular and histological characterization of bovine papillomavirus in North 349 West Italy. Vet Microbiol 180:113–117
Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hatama S, Ishihara R, Ueda Y, Kanno T, Uchida I (2011) Detection of a novel bovine papillomavirus type 11 (BPV-11) using xipapillomavirus consensus polymerase chain reaction primers. Arch Virol 156:1281–1285
Jarrett WF (1985) Bovine papillomaviruses. Clin Dermatol 3:8–19
Jarrett WFH, O’Neil BW, Gaukroger JM, Smith KT, Laird HM, Campo MS (1990) Studies on vaccination against papillomaviruses: the immunity after infection and vaccination with bovine papillomaviruses of different types. Vet Rec 126:473–475
Kumar P, Nagarajan N, Saikumar G, Arya RS, Somvanshi R (2013) Detection of bovine papilloma viruses in wart-like lesions of upper gastrointestinal tract of cattle and buffaloes. Transbound Emerg Dis 63:56–67
Leishangthem GD, Somvanshi R, Tiwari AK (2008) Detection of bovine papillomaviruses in cutaneous warts/papillomas in cattle. Indian J Vet Pathol 32:15–20
Lindsey CL, Almeida ME, Vicari CF, Carvalho C, Yaguiu A, Freitas AC, Becak W, Stocco RC (2009) Bovine papillomavirus DNA in milk, blood, urine, semen, and spermatozoa of bovine papillomavirus-infected animals. Genet Mol Res 8:310–318
Lunardi M, de Alcantara BK, Otonel RA, Rodrigues WB, Alfieri AF, Alfieri AA (2013) Bovine papillomavirus type 13 DNA in equine sarcoids. J Clin Microbiol 51:2167–2171
Lunardi M, Tozato CC, Alfieri AF, Alcantara BK, Vilas-Boas LA, Otonel RAA, Headley SA, Alfieri AA (2016) Genetic diversity of bovine papillomavirus types, including two putative new types, in teat warts from dairy cattle herds. Arch Virol 161:1569–1577
Maiolino PA, Ozkul A, Sepici-Dincel A, Roperto F, Yucel G, Russo V, Urraro C, Luca R, Riccardi MG, Martano M, Borzacchiello G, Esposito I, Roperto S (2013) Bovine papillomavirus type 2 infection and microscopic patterns of urothelial tumors of the urinary bladder in water buffaloes. Biomed Res Int 146:269–275
Maeda Y, Shibahara T, Wada Y, Kadota K, Kanno T, Uchida I, Hatama S (2007) An outbreak of teat papillomatosis in cattle caused by bovine papilloma virus (BPV) type 6 and unclassified BPVs. Vet Microbiol 121:242–248
Nasir L, Campo MS (2008) Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Vet Dermatol 19:243–254
Ogawa T, Tomita Y, Okada M, Schinozaki K, Kubonoya H, Kaiho I, Shirisawa H (2004) Broad-spectrum detection of papillomaviruses in bovine teat papillomas and healthy teat skin. J Gen Virol 85:2191–2197
Pangty K, Singh S, Goswami R, Saikumar G, Somvanshi R (2010) Detection of BPV-1 and -2 and quantification of BPV-1 by real-time PCR in cutaneous warts in cattle and buffaloes. Transbound Emerg Dis 57:185–196
Rai GK, Saxena M, Singh V, Somvanshi R, Sharma B (2011) Identification of bovine papilloma virus 10 in teat warts of cattle by DNase-SISPA. Vet Microbiol 147:416–419
Roperto S, Russo V, Ozkul A, Corteggio A, Sepici-Dincel A, Catoi C, Esposito I, Riccardi MG, Urraro C, Luca R, Ceccarelli DM, Longo M, Roperto F (2013) Productive infection of bovine papillomavirus type 2 in the urothelial cells of naturally occurring urinary bladder tumors in cattle and water buffaloes. PLoS One 8:e62227
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Santos EUD, Silva MAR, Pontes NE, Coutinho LCA, Paiva SSL, Castro RS, Freitas AC (2016) Detection of different bovine papillomavirus types and co-infection in bloodstream of cattle. Transbound Emerg Dis 63:103–108
Schmitt M, Fiedler V, Muller M (2010) Prevalence of BPV genotypes in a German cowshed determined by a novel multiplex BPV genotyping assay. J Virol Methods 170:67–72
Silva MS, Weiss M, Brum MCS, Anjos BL, Torres FD, Weiblen R, Flores EF (2010) Molecular identification of bovine papillomaviruses associated with cutaneous warts in southern Brazil. J Vet Diagn Invest 22:603–606
Silva MA, de Albuquerque BM, Pontes NE, Coutinho LC, Leitao MC, Reis MC, Castro RS, Freitas AC (2013) Detection and expression of bovine papillomavirus in blood of healthy and papillomatosis-affected cattle. Genet Mol Res 12:3150–3156
Silvestre O, Borzacchiello G, Nava D, Iovane D, Russo V, Vecchio D, D’Ausilio F, Gault EA, Campo MS, Paciello O (2009) Bovine papillomavirus type 1 DNA and E5 oncoprotein expression in water buffalo fibropapillomas. Vet Pathol 46:636–641
Singh V, Somvanshi R, Tiwari AK (2009) Papillomatosis in Indian cattle: occurance and etiopathology. Indian J Vet Pathol 33:52–57
Tamura K, Dudley J, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan MT, Yıldırım Y, Sozmen M, Bılge Dagalp S, Yılmaz V, Kırmızıgul AH, Gokce E (2012) A histopathological, ımmunohistochemical and molecular study of cutaneous bovine papillomatosis. Kafkas Univ Vet Fak Derg 18:739–744
Van Engelenburg FAC, Maes RK, Van Oirschot JT, Rijsewijk FAM (1993) Development of a rapid and sensitive polymerase chain reaction assay for detection of bovine herpesvirus type 1 in bovine semen. J Clin Microbiol 31:3129–3135
Yaguiu A, Carvalho C, Freitas AC, Góes LGB, Dagli MLZ, Birgel EH Jr, Stocco dos Santos RC (2006) Papilomatosis in cattle: In situ detection of bovine papilomavirus DNA sequences in reproductives tissues. Braz J Morphol 23:525–529
Yaguiu A, Dagli ML, Birgel EH Jr, Alves Reis BC, Ferraz OP, Pituco EM, Freitas AC, Becak W, Stocco RC (2008) Simultaneous presence of bovine papillomavirus and bovine leukemia virus in different bovine tissues: in situ hybridization and cytogenetic analysis. Genet Mol Res 7:487–497
Zhu W, Dong J, Shimizu E, Hatama S, Kadota K, Goto Y, Haga T (2012) Characterization of novel bovine papillomavirus type 12 (BPV-12) causing epithelial papilloma. Arch Virol 157:85–91
Acknowledgements
We thank to Vet.Ali Haciomeroglu for all helps.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Dagalp, S.B., Dogan, F., Farzanı, T.A. et al. The genetic diversity of bovine papillomaviruses (BPV) from different papillomatosis cases in dairy cows in Turkey. Arch Virol 162, 1507–1518 (2017). https://doi.org/10.1007/s00705-017-3258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3258-8