Abstract
Current influenza vaccines provide strain-specific protection against homologous subtypes and need to be updated annually. Therefore, it is essential to develop a universal vaccine that would induce broadly cross-protective immunity against homologous and heterologous influenza A viruses. The highly conserved HA2 subunit is a promising candidate for developing a universal influenza vaccine. Here, we hypothesized that the HA2 subunit could be displayed on the surface of Lactococcus lactis (L. lactis), using Spax as an anchor protein (L. lactis/pNZ8008-Spax-HA2) and that L. lactis/pNZ8008-Spax-HA2 would have immunogenicity by oral administration without the use of adjuvant in the mouse model. To address this hypothesis, we show that oral vaccination of mice with L. lactis/pNZ8008-Spax-HA2 elicited significant humoral and mucosal immune responses. Importantly, L. lactis/pNZ8008-Spax-HA2 provided 100 % protection against homologous H5N1 or heterologous H1N1 virus challenge. These results suggest that an HA2 subunit presented on the surface of L. lactis is an effective universal vaccine candidate against influenza A viruses in the poultry industry and in humans.
Similar content being viewed by others
Introduction
Influenza virus belongs to the family Orthomyxoviridae and is a lipid-enveloped virus with a segmented negative-sense single-stranded RNA genome. The envelope of the virion contains two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which are responsible for virus entry via attachment to host-cell sialic acid receptors and progeny release, respectively [1]. There are three types of influenza viruses, A, B, and C, and influenza A viruses are a major public-health concern worldwide [2].
Vaccination is the most effective way to prevent and control influenza A virus infection. However, most current vaccine strategies predominantly focus on raising a humoral response against hemagglutinin (HA) – the more abundant, immunodominant glycoprotein on the surface of the influenza virus [3], these vaccines mainly induce strain-specific neutralizing antibodies that target the highly variable regions of the globular head in the HA1 subunit, and therefore, they provide a very narrow breadth of protection [4–6]. Since influenza A viruses are able to evade human herd immunity by constantly changing the antigenic regions of HA, vaccines have to be reformulated annually based on surveillance data of circulating influenza virus strains and assessment of antigenic relatedness [5]. Thus, efforts are being undertaken to develop universal influenza A vaccines that induce cross-protective immunity. The highly conserved ion channel protein (M2) and the nucleoprotein (NP) of influenza viruses have been evaluated for the induction of cross-protective cellular immunity and viral clearance [7, 8]. Unfortunately, vaccines based on M2 or NP show poor immunogenicity [9]. Thus, there is an urgent need to develop another universal vaccine to confer broader protection against divergent influenza A viruses.
The HA protein is composed of HA1 and HA2 subunits. The HA1 subunit is highly immunogenic and therefore elicits high levels of neutralizing antibodies during natural infection, leading to clearance of the virus [9], but antibodies to HA1 typically do not provide cross-protection against divergent strains due to antigenic shift and/or drift [10]. On the other hand, the HA2 subunit, which represents most of the HA stem region, shows high sequence conservation among the different HA subtypes [11]. Antibodies directed against the highly conserved HA2 subunit are known to be broadly neutralizing in vitro and broadly protective in passive-transfer challenge in the mouse or ferret model [12–16]. Therefore, the HA2 subunit is a very attractive target for developing a broadly protective influenza vaccine [2, 17–19].
L. lactis is an ideal mucosal vaccine delivery vehicle [20, 21]. Our previous studies have shown that L. lactis expressing the HA or HA1 of avian influenza H5N1 virus is a safe and effective vaccine candidate against homologous H5N1 virus infection in the mouse, chicken or ferret model [22–25]. However, it remains largely unknown whether the highly conserved HA2 subunit based on recombinant L. lactis surface display technology in the absence of mucosal adjuvant can provide cross-protective immunity against divergent influenza A viruses in a mouse model.
In the present study, HA2 subunit derived from A/chicken/Henan/12/2004 (H5N1) was displayed on the surface of recombinant L. lactis in which Spax, derived from Staphylococcus aureus (S. aureus), was used as an anchor protein. Oral vaccination of mice with recombinant L. lactis/pNZ8008-Spax-HA2 induced a significant humoral and mucosal immune response. Most importantly, recombinant L. lactis/pNZ8008-Spax-HA2 provided complete protection against lethal challenge with homologous and heterologous influenza A viruses. These findings suggest that recombinant L. lactis/pNZ8008-Spax-HA2 without the use of mucosal adjuvant can be considered an alternative vaccine candidate for use during outbreaks of influenza A virus infection.
Materials and methods
Viruses
A/chicken/Henan/12/2004 (H5N1) and A/Puerto Rico/1/34(H1N1) viruses were kindly provided by the Institute of Virology, Chinese Academy of Science (Wuhan, China). Viral isolates were identified by hemagglutination assay after inoculation into the allantoic cavity of 10-day-old specific-pathogen-free (SPF) chicken embryos. Three days after inoculation, allantoic fluids from infected eggs were harvested and stored at -80 °C. The 50 % embryo lethal dose (ELD50) was determined for each stock, and the viruses were subsequently isolated in a biosafety level 3 (BSL-3) facility. To prepare the adapted viruses in mice, lung-to-lung passages were performed. Briefly, BALB/c mice were anesthetized and inoculated with 20 µL of H5N1 or H1N1 viral suspension by intranasal drip. On the third day after inoculation, the mice were sacrificed and their tracheae and lungs were collected and washed three times with a total of 2 mL of phosphate-buffered saline (PBS) containing 0.1 % bovine serum albumin (BSA). The bronchoalveolar washes were used to infect the next group of mice after removing cellular debris by centrifugation. The 50 % mouse lethal dose (MLD50) was determined using the Reed-Muench method [26].
Construction of L. lactis/pNZ8008-Spax-HA2
The SpaX anchor domain (411 bp) was amplified by PCR from the Staphylococcus aureus (S. aureus) genome using the primers S-F (5′ CTAGCTAGCAGTCTTCTAACCGAG 3′; NheI sequence underlined) and S-R (5′ CCGCCGCCGCCGCGGCTTACTCCAGCTCTAT 3′; GS linker sequence underlined). Similarly, an HA2 gene fragment (744 bp) from A/chicken/Henan/12/2004 (H5N1) (Clade 8.0) was amplified by PCR from pcDNA3.1-HA (kindly provided by the Institute of Virology, Chinese Academy of Science, Wuhan, China) using the primers H-F (5′ GGCGGCGGCGGCGCCGAGGAGGACTATTTG 3′; GS sequence underlined) and H-R (5′ CCGAATTCTTAACTACAATCTGAACTC 3′; EcoR I sequence underlined). The Spax and HA2 fragments were fused via the GS linker using primers S-F and H-R to make Spax-HA2. The resulting Spax-HA2 containing Nhe I/EcoR I sites was subcloned into the L. lactis expression vector pNZ8008 (Fig. 1A) and then introduced into competent L. lactis NZ9000 by electroporation. A positive clone of L. lactis/pNZ8008-Spax-HA2 was selected and expressed as described previously [22]. L. lactis/pNZ8008-Spax was used as a negative control for subsequent tests.
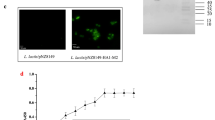
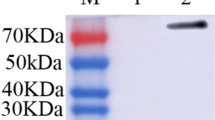
Expression of HA2 displayed on the surface of L. lactis. (A) Schematic diagram of L. lactis/pNZ8008-Spax-HA2. (B) Western blot analysis. Lane 1, L. lactis-pNZ8008-Spax-HA2; Lane 2, L. lactis/pNZ8008-Spax. (C) Immunofluorescence microscopy assay of HA2 protein. L. lactis/pNZ8008-Spax (left) and L. lactis/pNZ8008-Spax-HA2 (right) (magnification: 1, 000×). (D) Flow cytometric analysis of HA2 displayed on the surface of L. lactis. L. lactis/pNZ8008-Spax (left) and L. lactis/pNZ8008-Spax-HA2 (right)
Western blot analysis
HA2 antigen expression on the surface of recombinant L. lactis was detected by Western blot analysis as described previously [22]. Briefly, 105 cells of L. lactis/pNZ8008-Spax-HA2 pellets were washed three times with 500 µL of sterile saline and then resuspended with 50 µL of 6× loading buffer and boiled for 10 minutes. Treated samples were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, California, USA). After blocking with 5 % non-fat milk at room temperature for 2 h, the membrane was incubated with a 1:500-diluted mouse monoclonal anti-HA antibody (kindly provided by Institute of Virology, Chinese Academy of Science, Wuhan, China). After overnight incubation at 4 °C, the membrane was incubated with affinity-purified horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1:5000 dilution) (Sigma-Aldrich Corporation, St. Louis, MO, USA). Finally, the membrane was allowed to react with West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA) and imaged using a Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence assay and flow cytometric analysis
The presence of HA2 protein displayed on the surface of L. lactis was confirmed by immunofluorescence assay using a Leica DM4000B fluorescence microscope and flow cytometry (FACS) analysis using a BD FacsCalibur (BD Bioscience, San Jose, CA, USA). Briefly, 105 cells of L. lactis/pNZ8008-Spax-HA2 were washed three times with sterile saline containing 0.5 % bovine serum albumin (BSA), incubated the monoclonal mouse anti-HA antibody at 4 °C for 1h, followed by 1:5000-diluted FITC-conjugated goat anti-mouse IgG (R&D Systems, USA) at 4 °C for 30 min, and resuspended with 500 µL of sterile saline. Finally, 5 µL of L. lactis/pNZ8008-Spax-HA2 cells was used for immunofluorescence assay and 300 µL of L. lactis/pNZ8008-Spax-HA2 cells was used for FACS analysis. L. lactis/pNZ8008-Spax cells were used as a negative control.
Animals, vaccine, immunization and sample collection
Six-week-old female BALB/c mice were used (SLC Company, Shanghai, China) in this study and housed in cages ventilated under negative pressure with HEPA-filtered air.
The concentration of recombinant L. lactis/pNZ8008-Spax-HA2 was adjusted with sterile saline to 1012 colony-forming units (CFU)/mL and stored at 4 °C until use. The mice (16 per group) were vaccinated orally with 500 µL of L. lactis/pNZ8008-Spax-HA2 at days 1, 2, 3 for prime immunization and days 17, 18, 19 for boost immunization. Saline and L. lactis/pNZ8008-Spax were used as controls.
On days 16 and 33 after the first immunization, blood samples were collected from the retro-orbital plexus. Sera were separated by centrifugation of blood at 2000 × g for 10 min and stored at -20 °C until use. Intestines (3 per group) were isolated from the vaccinated mice and washed with 500 µL of sterile saline.
Two weeks after the last immunization, all of the vaccinated mice were transferred to an animal BSL-3 containment facility and challenged intranasally with 20 µL of a viral suspension containing 5 × LD50 of A/chicken/Henan/12/2004 (H5N1) or A/Puerto Rico/1/34(H1N1) virus. The mice were monitored for 14 days and body weight loss and the survival rate after virus challenge were determined. Lungs (3 per group) were isolated from the vaccinated mice on day 3 post-challenge as described previously [23].
All animal immunizations were carried out at biosafety level 2 (BLS-2), and virus challenge experiments were performed in biosafety level 3 (BSL-3) containment facilities, in compliance with the Guidelines for Use and Care of Experimental Animals. The study was approved by the Animal Committee of the Institute of Nanchang University.
Enzyme-linked immunosorbent assay (ELISA)
HA2-specific IgG in sera and IgA in intestinal washes and feces were detected by (ELISA) using recombinant HA protein of A/chicken/Henan/12/2004 (H5N1) or A/Puerto Rico/1/34(H1N1) (2 µg/ml) as a coating antigen (kindly provided by the Institute of Virology, Chinese Academy of Science, Wuhan, China) as described previously [22, 23]. Optical density (OD) was measured at 405 nm using an ELISA plate reader. The IgG or IgA titer was defined as the lowest dilution with an OD greater than the mean OD of naïve controls plus two standard deviations. A value less than 25 was considered to be no significance.
Microneutralization assay
Microneutralizing antibody in the serum was determined as described previously [23]. Briefly, serial twofold dilutions of sera treated with receptor-destroying enzyme (RDE) from Vibro cholerae were mixed and incubated with a 35-μL suspension containing 100 times the 50 % tissue culture infective dose (TCID50) of H5N1 virus and then added to Madin-Darby canine kidney (MDCK) cells and incubated for 1 h. The H5N1-virus-infected MDCK cells were then cultured for 72 h at 37 °C in the presence of 5 % CO2, and the neutralizing titer was determined by hemagglutination test. For the HA test, 50 μl of 0.5 % rooster red blood cells was added to 50 μl of cell culture supernatant and incubated at room temperature for 30 min. The TCID50 was determined by the Reed-Muench method [26]. The neutralization titer (IC50) was defined as the reciprocal of the antiserum dilution at which H5N1 virus entry was inhibited by 50 %.
Statistical analysis
Differences between groups were analyzed by analysis of variance (ANOVA), and means were compared by Student’s t-test. P-values less than 0.05 were regarded as significant.
Results
HA2 protein expressed on the surface of L. lactis
The HA2 protein of A/chicken/Henan/12/2004 (H5N1) was fused with the C-terminal end of Spax through a GS linker (Fig. 1A). The HA2 protein expressed on L. lactis could be recognized by a mouse anti-HA monoclonal antibody, as clearly indicated by Western blotting (Fig. 1B). The expected size for the Spax-HA2 fusion protein was approximately 40 kDa.
To confirm that the HA2 protein was expressed on the surface of L. lactis, L. lactis/pNZ8008-Spax-HA2 cells were incubated with mouse anti-HA monoclonal antibody and then reacted with FITC-conjugated goat anti-mouse IgG for detection by immunofluorescence assay and FACS analysis. As shown in Fig. 1C and D, L. lactis/pNZ8008-Spax-HA2 gave a high specific and positive signal. In contrast, L. lactis/pNZ8008-Spax cells did not give a positive signal. These results demonstrated that the HA2 protein was located on the surface of L. lactis.
Antibody responses induced by L. lactis/pNZ8008-Spax-HA2
HA2-specific antibody responses were measured by ELISA. As shown in Fig. 2A and B, serum IgG titers did not differ significantly between the groups 16 days after the first immunization. However, a highly significant increase was observed in the L. lactis/pNZ8008-Spax-HA2 group 33 days after the first immunization, whereas there were still no significant changes in the saline or L. lactis/pNZ8008-Spax group after the boost immunization.
Antibody responses elicited by L. lactis/pNZ8008-Spax-HA2 in mice. Sera and intestinal washes were collected from mice vaccinated orally with saline, L. lactis/pNZ8008-Spax or L. lactis/pNZ8008-Spax-HA2. (A) HA2-specific IgG antibody responses in sera using HA of A/chicken/Henan/12/2004 (H5N1) as a coating antigen. (B) HA2-specific IgG antibody responses in sera using HA of A/Puerto Rico/1/34(H1N1) as a coating antigen. (C) HA-specific IgA antibody responses in intestinal washes (3 per group) using HA of A/chicken/Henan/12/2004 (H5N1) as a coating antigen. (D) HA-specific IgA antibody responses in intestinal washes (3 per group) using HA of A/Puerto Rico/1/34(H1N1) as a coating antigen. Data are presented as mean ± SD. An asterisk indicates a significant difference compared to saline and L. lactis-pNZ8008-Spax controls (p < 0.05)
Similarly, no significant IgA titers were measured in any of the groups on day 16. Significantly, mucosal IgA antibody titers were detected on day 33 only in the intestinal washes of mice vaccinated orally with L. lactis/pNZ8008-Spax-HA2 (Fig. 2B, C). These results revealed that L. lactis/pNZ8008-Spax-HA2 could induce significant humoral and mucosal immune responses in vaccinated mice after prime-boost immunization.
A, miccroneutralization assay revealed that there was no significant neutralizing antibody response in any of the groups on day 16 and day 33 after the first immunization (Table 1).
Taken together, mice vaccinated orally with L. lactis/pNZ8008-Spax-HA2 after the boost immunization produced cross-reactive antibodies against different influenza A viruses, which might contribute to preventing virus infection. However, HA2 presented on L. lactis surface did not show neutralizing reactivity.
Cross-protection against different influenza A viruses
To investigate whether L. lactis/pNZ8008-Spax-HA2 could provide cross-protective immunity against divergent influenza A viruses, all vaccinated mice were challenged with 100 TCID50 of mouse-adapted A/chicken/Henan/12/2004 (H5N1) or A/Puerto Rico/1/34(H1N1) and monitored for 14 days. The control groups that received saline or L. lactis/pNZ8008-Spax showed clinical signs of severe disease, significant body weight loss, and a higher virus titer in the lung, starting on day 2 after virus infection, and they died within 8 days after lethal challenge (Fig. 3). In contrast, mice vaccinated orally with L. lactis/pNZ8008-Spax-HA2 were protected completely against virus challenge; all surviving mice experienced mild weight loss and a lower virus titer in the lung (Fig. 3). Collectively, the results of this study provide reliable evidence that L. lactis/pNZ8008-Spax-HA2 is a safe and effective vaccine candidate for inducing cross-protective immunity against homologous and heterologous influenza A viruses in a mouse model.
Cross-protection of L. lactis/pNZ8008-Spax-HA2 against lethal challenge with divergent influenza A viruses. The results are expressed in terms of percent body weight (A and B), lung virus titer (C and D) and percent survival (E and F) (10 mice per group). Two weeks after the last immunization, mice were infected intranasally with 20 µL of 5 times the LD50 of A/chicken/Henan/12/2004 (H5N1) (A, C and E) or A/Puerto Rico/1/34(H1N1) (B, D and F) (10 mice per group). The data for lung virus titers (3 mice per group) are indicated as mean ± SD. An asterisk indicates a significant difference compared with saline and L. lactis/pNZ8008-Spax controls (p < 0.05)
Discussion
A major obstacle in vaccine development against influenza A virus infection is the rapid evolution of genetic diversity [27]. As a result, there is an urgent need to develop a universal influenza vaccine to confer broader protection against divergent influenza A viruses. In this study, we investigated the cross-protective immunity of the highly conserved HA2 subunit presented on the surface of L. lactis, using Spax as an anchor protein. This was tested in a mouse model via oral administration without the use of mucosal adjuvant.
In a previous study, we showed that L. lactis/pNZ8110-pgsA-HA1, in which pgsA was used as an anchor protein, combined with cholera toxin subunit B (CTB) as an adjuvant, provided immune protection against the homologous H5N1 virus in a mouse model [22]. In that study, the level of pgsA displayed on the cell surface was relatively low, requiring a high dosage for oral administration. As an alternative anchor protein, Spax, derived from Staphylococcus aureus (S. aureus) was designed to display heterologous proteins [28]. There have been no attempts to investigate the immune efficacy of recombinant L. lactis in which Spax is used as an anchor. In this study, we used the highly conserved HA2 subunit as a model antigen and constructed a novel display system based on the fusion of Spax and HA2 through a GS linker. L. lactis/pNZ8008-Spax-HA2 showed a relatively high efficiency for displaying antigen (Fig. 1C, D). These findings imply that Spax is suitable as an alternative anchor protein for displaying foreign proteins for influenza vaccine development.
It is critical to determine the optimal dosage for oral administration of a vaccine. However, different L. lactis constructs have different immune efficacy in different animal models. The purpose of this study was to investigate the immunogenicity of L. lactis/pNZ8008-Spax-HA2 without the use of mucosal adjuvant. In this case, the use of repeated immunizations should be an option to enhance the immunogenicity in the mouse model. The present study demonstrated that oral vaccination of mice with L. lactis/pNZ8008-Spax-HA2 elicited significant serum IgG and mucosal IgA antibody responses against different influenza A virus HAs (Fig. 2). Recent studies have shown that antibodies against the highly conserved HA2 have broad neutralizing activity, which may play an important role in recovery from viral infection [29, 30]. In this study, the highly conserved HA2 subunit displayed on the surface of L. lactis induced significant humoral and mucosal immune responses, which might contribute to providing cross-protection against influenza A viruses.
Virus challenge is the standard way to evaluate the efficacy of a new influenza vaccine [3]. We tested whether HA2 presented on the L. lactis surface would be able to provide cross-protection against divergent influenza A viruses. Mice immunized orally with L. lactis/pNZ8008-Spax-HA2 showed mild body weight loss and lower virus shedding in the lungs after virus challenge (Fig. 3). Most importantly, mice in the L. lactis/pNZ8008-Spax-HA2 group were completely protected against lethal challenge with homologous H5N1 virus and heterologous H1N1 or H3N2 virus. These findings are in agreement with those of Margine et al. [4], who reported that a vaccination strategy based on the HA2 subunit of H3 hemagglutinin (group 2) induced broadly neutralizing anti-stalk antibodies that were highly cross-reactive with heterologous H3, H10, H14, H15, and H7 hemagglutinins and conferred broad protection against influenza. Taken together, these studies suggest that the highly conserved HA2 subunit presented on the L. lactis surface could potentially be used in the development of a universal influenza vaccine that would contribute to human health, as well as being applicable to the poultry industry.
In summary, the highly conserved HA2 subunit represents a new research target for a novel universal influenza vaccine. This is the first report showing that an HA2 subunit displayed on the surface of L. lactis has the potential to induce significant cross-protective immunity against homologous and heterologous influenza A viruses in a mouse model. Therefore, L. lactis display technology can serve as a platform for development of a universal influenza vaccine that confers broad-spectrum protection against divergent influenza A viruses in poultry and humans.
References
Fiege JK, Langlois RA (2015) Investigating influenza A virus infection: tools to track infection and limit tropism. J Virol 89:6167–6170
Pleschka S (2013) Overview of influenza viruses. Curr Top Microbiol Immunol 370:1–20
Wong SS, Webby RJ (2013) Traditional and new influenza vaccines. Clin Microbiol Rev 26:476–492
Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F (2013) H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737
Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF (2011) H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797
Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC (2008) Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671
Cianci C, Gerritz SW, Deminie C, Krystal M (2014) Influenza nucleoprotein: promising target for antiviral chemotherapy. Viruses 6:3159–3180
Reese KA, Lupfer C, Johnson RC, Mitev GM, Mullen VM, Geller BL, Pastey M (2013) A novel lactococcal vaccine expressing a peptide from the M2 antigen of H5N2 highly pathogenic avian influenza A virus prolongs survival of vaccinated chickens. Vet Med Int 2013:316926
Zhang H, Wang L, Compans RW, Wang BZ (2014) Universal influenza vaccines, a dream to be realized soon. Viruses 6:1974–1991
Prabhu N, Prabakaran M, Ho HT, Velumani S, Qiang J, Goutama M, Kwang J (2009) Monoclonal antibodies against the fusion peptide of hemagglu-tinin protect mice from lethal influenza A virus H5N1 infection. J Virol 83:2553–2562
Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA (2009) Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251
Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J (2011) A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850
Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, García-Sastre A, Albrecht RA (2014) Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442
Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA (2009) Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16:265–273
Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P (2012) A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188
Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM (2010) Palese P. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 6:e1000796
Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, ter Meulen J, Liang X, Varadarajan R (2010) Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci USA 107:13701–13706
Corti D, Suguitan AL Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A (2010) Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest 120:1663–1673
Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, García-Sastre A, Palese P (2010) Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 1. pii e00018–10
Bahey-El-Din M (2012) Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine 30:685–690
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362
Lei H, Sheng Z, Ding Q, Chen J, Wei X, Lam DM, Xu Y (2011) Evaluation of oral immunization with recombinant avian influenza virus HA1 displayed on the Lactococcus lactis surface and combined with the mucosal adjuvant cholera toxin subunit B. Clin Vaccine Immunol 18:1046–1051
Lei H, Xu Y, Chen J, Wei X, Lam DM (2010) Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology 407:319–324
Lei H, Peng X, Shu H, Zhao D (2015) Intranasal immunization with live recombinant Lactococcus lactis combined with heat-labile toxin B subunit protects chickens from highly pathogenic avian influenza H5N1 virus. J Med Virol 87:39–44
Lei H, Peng X, Ouyang J, Zhao D, Jiao H, Shu H, Ge X (2015) Intranasal immunization of recombinant Lactococcus lactis induces protection against H5N1 virus in ferrets. Virus Res 196:56–59
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497
Linster M, van Boheemen S, de Graaf M, Schrauwen EJ, Lexmond P, Mänz B, Bestebroer TM, Baumann J, van Riel D, Rimmelzwaan GF, Osterhaus AD, Matrosovich M, Fouchier RA (2014) Herfst S (2014) Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157:329–339
Song D, Gu Q (2009) Surface expression of Helicobacter pylori urease subunit B gene E fragment on Lactococcus lactis by means of the cell wall anchor of Staphylococcus aureus protein A. Biotechnol Lett 31:985–989
Lee JS, Chowdhury MY, Moon HJ, Choi YK, Talactac MR, Kim JH, Park ME, Son HY, Shin KS, Kim CJ (2013) The highly conserved HA2 protein of the influenza A virus induces a cross protective immune response. J Virol Methods 194:280–288
Khanna M, Sharma S, Kumar B, Rajput R (2014) Protective immunity based on the conserved hemagglutinin stalk domain and its prospects for universal influenza vaccine development. Biomed Res Int 2014:546274
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31360225) and Natural Science Foundation of Jiangxi Province (No. 20114BAB214014) to H. Lei.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict interest.
Rights and permissions
About this article
Cite this article
Lei, H., Peng, X., Zhao, D. et al. Cross-protection of Lactococcus lactis-displayed HA2 subunit against homologous and heterologous influenza A viruses in mice. Arch Virol 160, 3011–3019 (2015). https://doi.org/10.1007/s00705-015-2587-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2587-8