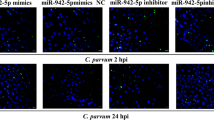
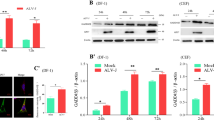
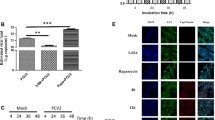
Abstract
In the present study, we characterized the pathways by which a laboratory-attenuated vesicular stomatitis virus (La-VSV) induces apoptosis in BHK cells. It was found that La-VSV induced a loss of mitochondrial membrane potential (ΔΨm) and activated caspase-9 and -3, but not caspase-8, indicating that the induction of apoptosis by La-VSV may involve an intrinsic apoptotic pathway. Although aberrant expression of microRNAs (miRNAs) has been linked to viral infection, little is known about changes in the cellular miRNA expression profile following VSV infection. Here, we attempted to identify miRNA expression profiles in VSV-infected BHK cells using miRNA microarray. Data analysis revealed that 28 miRNAs consistently responded to VSV-infection, 12 of which were down-regulated and 16 of which were up-regulated. miR-146a of these miRNAs has been found to be up-regulated in LPS-stimulated monocytes and VSV-infected macrophages, suggesting that VSV-induced miR-146a expression occurs not only in immune cells but also in other host cells. We further found that miR-706 inhibited VSV-induced apoptosis by decreasing caspase-3 and -9 activation, suggesting that induction of miR-706 expression may be a novel strategy for survival of VSV, allowing it to escape the apoptosis response of the host. In summary, our results indicate that miRNAs might play important roles in VSV infection and that their aberrant expression could be involved in VSV pathogenesis.






Similar content being viewed by others
References
Jacobson MD, Weil M, Raff MC (1997) Programmed cell death in animal development. Cell 88:347–354
Roulston A, Marcellus RC, Branton PE (1999) Viruses and apoptosis. Annu Rev Microbiol 53:577–628
Gougeon ML, Olivier R, Garcia S, Guetard D, Dragic T, Dauguet C, Montagnier L (1991) Demonstration of an engagement process towards cell death by apoptosis in lymphocytes of HIV infected patients. C R Acad Sci III 312:529–537
Moriishi K, Koura M, Matsuura Y (2002) Induction of Bad-mediated apoptosis by Sindbis virus infection: involvement of pro-survival members of the Bcl-2 family. Virology 292:258–271
Desprès P, Frenkiel MP, Ceccaldi PE, Duarte Dos Santos C, Deubel V (1998) Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol 72:823–829
Sumikoshi M, Hashimoto K, Kawasaki Y, Sakuma H, Suzutani T, Suzuki H, Hosoya M (2008) Human influenza virus infection and apoptosis induction in human vascular endothelial cells. J Med Virol 80:1072–1078
Lichty BD, Power AT, Stojdl DF, Bell JC (2004) Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med 10:210–216
Bi Z, Barna M, Komatsu T, Reiss CS (1995) Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol 69:6466–6472
Balachandran S, Porosnicu M, Barber GN (2001) Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol 75:3474–3479
Koyama AH (1995) Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res 37:285–290
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Cullen BR (2004) Transcription and processing of human microRNA precursors. Mol Cell 16:861–865
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Lai EC (2005) miRNAs: whys and wherefores of miRNA-mediated regulation. Curr Biol 15:R458–R460
Gottwein E, Cullen BR (2008) Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3:375–387
Umbach JL, Cullen BR (2009) The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev 23:1151–1164
Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, Das SC, Pattnaik AK, Beutler B, Han J (2007) Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 27:123–134
Kopecky SA, Willingham MC, Lyles DS (2001) Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol 75:12169–12181
Yoshida M (2001) Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol 19:475–496
Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G (2003) Poxviruses and immune evasion. Annu Rev Immunol 21:377–423
Everett H, McFadden G (1999) Apoptosis: an innate immune response to virus infection. Trends Microbiol 7:160–165
Benedict CA, Norris PS, Ware CF (2002) To kill or be killed: viral evasion of apoptosis. Nat Immunol 3:1013–1018
Hobbs JA, Hommel-Berrey G, Brahmi Z (2003) Requirement of caspase-3 for efficient apoptosis induction and caspase-7 activation but not viral replication or cell rounding in cells infected with vesicular stomatitis virus. Hum Immunol 64:82–92
Gadaleta P, Vacotto M, Coulombié F (2002) Vesicular stomatitis virus induces apoptosis at early stages in the viral cycle and does not depend on virus replication. Virus Res 86:87–92
Desforges M, Despars G, Bérard S, Gosselin M, McKenzie MO, Lyles DS, Talbot PJ, Poliquin L (2002) Matrix protein mutations contribute to inefficient induction of apoptosis leading to persistent infection of human neural cells by vesicular stomatitis virus. Virology 295:63–73
Kopecky SA, Lyles DS (2003) Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol 77:4658–4669
Gaddy DF, Lyles DS (2007) Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol 81:2792–2804
Pearce AF, Lyles DS (2009) Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol 83:9102–9112
Gadaleta P, Perfetti X, Mersich S, Coulombié F (2005) Early activation of the mitochondrial apoptotic pathway in vesicular stomatitis virus-infected cells. Virus Res 109:65–69
Gaddy DF, Lyles DS (2005) Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol 79:4170–4179
Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN (2000) Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol 74:1513–1523
Zhao JJ, Sun DG, Wang J, Liu SR, Zhang CY, Zhu MX, Ma X (2008) Retinoic acid downregulates microRNAs to induce abnormal development of spinal cord in spina bifida rat model. Childs Nerv Syst 24:485–492
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283:1026–1033
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27:435–448
Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT (2007) Altered microRNA expression in human heart disease. Physiol Genomics 31:367–373
Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M (2007) Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315:1579–1582
Cameron JE, Fewell C, Yin Q, McBride J, Wang X, Lin Z, Flemington EK (2008) Epstein–Barr virus growth/latency III program alters cellular microRNA expression. Virology 382:257–266
Wang FZ, Weber F, Croce C, Liu CG, Liao X, Pellett PE (2008) Human cytomegalovirus infection alters the expression of cellular microRNA species that affect its replication. J Virol 82:9065–9074
Yeung ML, Bennasser Y, Myers TG, Jiang G, Benkirane M, Jeang KT (2005) Changes in microRNA expression profiles in HIV-1-transfected human cells. Retrovirology 2:81
Houzet L, Yeung ML, de Lame V, Desai D, Smith SM, Jeang KT (2008) MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 5:118
Tamminga J, Kathiria P, Koturbash I, Kovalchuk O (2008) DNA damage-induced upregulation of miR-709 in the germline downregulates BORIS to counteract aberrant DNA hypomethylation. Cell Cycle 7:3731–3736
Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y (2008) hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res 1236:185–193
Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X (2009) MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 183:2150–2158
Acknowledgments
Microarray experiments were performed by Kangchen Bio-tech, Shanghai, China. This study was supported by an “innovative start-up” project of Veterinary Research Institute, Academy of Military Medical Sciences (Project No. YCX0902).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lian, H., Liu, W., Liu, Q. et al. A laboratory-attenuated vesicular stomatitis virus induces apoptosis and alters the cellular microRNA expression profile in BHK cells. Arch Virol 155, 1643–1653 (2010). https://doi.org/10.1007/s00705-010-0749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-010-0749-2