Abstract
Accumulated evidence has demonstrated abnormal amygdala activation in bipolar disorder (BD). The olfactory bulb (OB) has vigorous connections with the amygdala. Although odor-related functions of the OB decreased during the evolutionary process, we hypothesized that an evolved OB with increased activation in emotion regulation may be one of the main factors affecting amygdala functions in BD. Our aim was to investigate metabolism in the OB and amygdala in patients with BD. Twenty-six patients diagnosed with BD according to DSM-5 diagnostic criteria were included in this cross-sectional study. Metabolism in the OB and amygdala was assessed using fluorodeoxyglucose positron emission tomography/CT in patients with BD. The OB and amygdala metabolism was compared with the patients’ Z scores. Both OB and amygdala metabolic activities were significantly higher than in the controls. A positive correlation was detected between right/left amygdala metabolism and right OB metabolism (p < 0.05, r:467 and r:662, respectively). This study increased our understanding of the etiopathogenesis of BD. In BD, the main cause of hypermetabolism in the amygdala may be increased metabolism in the OB. During evolution, the OB may have assumed a dominant role in emotional processing rather than olfactory functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar disorder is a chronic disease characterized by mood dysregulation and cognitive impairment, accompanied by neuropathologies in specific brain regions. Genetic predisposition is an important risk factor in bipolar disorder (Hough and Ursano 2006) and determining objective biomarkers, such as structural and functional brain abnormalities in BD, has the potential to provide early diagnosis and a better understanding of the pathophysiology of the disease. Patients with BD are reported to show hypoactivation in brain regions related to the cortico-cognitive pathway (such as the ventrolateral prefrontal cortex) and in regions related to emotional clarity (such as the amygdala)(Houenou et al. 2011). This imbalance between the two systems is thought to cause periods of mania and depression (Blumberg et al. 2002; Spielberg et al. 2016). In particular, BD has been associated with amygdala activity and a lack of global network communication in various brain regions (Spielberg et al. 2016).
The amygdala has received particular attention because of its central role in the emotional system (Sah et al. 2003). The amygdala controls emotions such as hunger, satiety, sexual desire, reproductive instinct, and fight or flight. It also plays a key role in fear, pleasure, and affect and in the emotional evaluation of sensory stimuli, emotional learning, memory, and affective disorders, especially anxiety (McGaugh 2004). However, the olfactory bulb (OB), which is responsible for the sense of smell, has direct and indirect connections with the hippocampus, anterior cingulate cortex, insular region, thalamus, hypothalamus, and orbitofrontal cortex, and particularly the amygdaloid nucleus. Together, this network of connections is responsible for the effect of smells on the regulation of behavior, nutrition, emotion, autonomic states, and memory (Gottfried 2006). Some of these structures also send intense feedback to the OB (Carmichael et al. 1994). We believe that the functional and structural defects of the OB directly affect these regions and are responsible for the etiopathogenesis of BD. However, no clinical studies have yet revealed a relationship between BD and the OB.
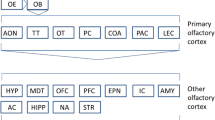
The structures that process emotions and odors are thought to have evolved together (Northcutt 2002). The olfactorious structure lost many functions throughout evolution; however, its functions in puberty and reproduction are still preserved (Hughes et al. 2014). The OB has been shown to play an important role in reproduction in many animal studies, and its role in humans is underestimated (Jouhanneau et al. 2014; Szymanski and Keller 2014). For this reason, its possible role in BD may have been overlooked. Odor processing has a relatively poor temporal and spatial resolution compared with other senses, such as vision or hearing. One reason why this poorly understood sense is so highly preserved throughout the evolutionary process might be that the sense of smell performs key functions in behavioral adaptation and emotion regulation (Croy and Hummel 2017). Therefore, the sense of smell may have played a more dominant role in emotional processing in the evolutionary process than is presently recognized. Consequently, the OB functions may have been increased in terms of their relevance for emotional processing (Figs. 1 and 2).
Interestingly, bipolar patients show a significant deterioration in olfactorious identification. In recent years, many studies have been conducted on the olfactorious system in patients with neuropsychiatric disorders because of the recognition of the overlap between brain structures, such as the limbic system that creates emotional stimuli, and the olfactorious structures in the brain (Mesholam et al. 1998; Turetsky et al. 2012). These functional disorders that disrupt emotional processing also affect olfactory functions; consequently, olfactory dysfunction is a prodromal symptom in several neurodegenerative diseases (Doty et al. 1988; Brewer et al. 2003).
Of the afferent connections of the amygdala, only the olfactorious fibers are well defined anatomically. Neurophysiological studies conducted in humans and animals have shown that the amygdala responds strongly to olfactorious stimulation (Gottfried 2006). The olfactorious parts of the amygdala, in turn, provide direct input to the thalamus, hypothalamus, basal ganglia, and prefrontal cortex, as well as returning the projection to the OB (Gottfried 2006). The amygdala, therefore, communicates with the cortex via both the visual and olfactorious systems. The amygdala is at the forefront of communication between the temporal and orbitofrontal cortex in the visual system, but it provides communication between the OB and the olfactorious cortex in the olfactorious system. In patients with BD, the imbalance detected between the amygdala and the prefrontal (orbitofrontal) cortex may also exist between the olfactorious system and the amygdala.
The euthymic period in BD generally describes a stable mental health condition in which BD patients show no depression or manic episodes. However, patients with euthymic BD who do not show any clinical presentation may still experience difficulties in their adaptive skills and functionality, and may be unable to fully return to their pre-disease functionality (Liu et al. 2014). However, the euthymic period and its underlying causes remain poorly understood. Some neuroimaging studies have shown abnormal blood oxygenation level dependent (BOLD) activity in the amygdala, orbitofrontal cortex (OFC), and medial prefrontal cortex (mPFC) in response to emotional stimuli during remission in BD (Abler et al. 2008; Wesley et al. 2018). More specifically, abnormal elevation of the BOLD signal of the amygdala has been observed in patients with euthymic BD in response to fearful facial expressions (Blumberg et al. 2005; Foland et al. 2008). These abnormalities may arise due to the underlying causes of deficiencies in emotional and cognitive integration in bipolar I euthymia (Lawrence et al. 2004).
Detecting BD at an early stage is important for preventing the devastating consequences of this disorder; therefore, identifying neuromarkers for BD is invaluable. During the restrictions imposed by the recent COVID-19 pandemic, patients were prevented from reaching hospitals, and the diagnosis of newly diagnosed patients was delayed (Thome et al. 2021). The COVID-19 quarantine also affected the physical, mental, emotional, and social states of individuals with existing psychiatric illnesses (Odone et al. 2020). In addition to the lack of access to treatment, the decrease in physical activity among these patients caused an increase in their psychological symptoms. Notably, fewer depressive symptoms and suicidal thoughts were reported in those who did not have access to medication but who exercised (Belvederi Murri et al. 2019). For this reason, catching diseases like BD at an early stage is important not only for drug treatment but also for initiating this type of intervention.
In this study, we used fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT to investigate metabolism in the OB and amygdala of patients with BD. We hypothesized that, in BD, both the OB and amygdala and the connections between them contribute to the pathophysiology of the disorder. We believe that our study findings will contribute to the literature, as this is the first study to evaluate OB metabolism and its relationship in patients with BD.
Methods
This prospective study was carried out in patients in the euthymic period (Hamilton Depression Scale [HAM-D] < 7; Young Mania Rating Scale [YMRS] < 5) who applied to Gaziantep University Hospital Psychiatry Polyclinic between January 2020 and January 2021 and were followed up with the diagnosis of BD according to DSM-5 diagnostic criteria. SCID 2 compatible with DSM-5 was used in the interview (First 2014). The study consisted of 26 patients who met the inclusion criteria.
The psychiatric diagnoses of the 26 people were determined by performing a mental state examination, and each diagnosis and additional diagnoses were determined according to DSM-5. Follow-up notes and medical records of the participants were examined, face-to-face interviews were conducted with the relatives and the participants, and a sociodemographic data form, HAM-D, and YMRS scales were filled out based on the information provided. Approval for the research was obtained from the ethics committee of Gaziantep University with decision number 2019/515.
Participants
The impact size (d = 0.35) was chosen for the study to obtain statistically significant data regarding metabolism in the OB and amygdala in BD patients, and the minimum number of participants was determined to be 26 (α = 0.05, 1-β = 0.80). The analysis was performed using G power version 3.9.1. The 26 selected patients were in the euthymic period and met the inclusion criteria for participation in this study. Interview scales of the BD patients who came to their routine follow-ups within a 1 month interval were applied, and PET imaging was performed the next day by including them in the study according to their scale scores. Inclusion criteria were: (a) a diagnosis of BD according to DSM-5 diagnostic criteria; (b) age between 18 and 35 years; (c) voluntary acceptance to participate in study; and (d) in the euthymic period at the last control examination (HAM-D score below 7 and YMRS score below 5). Exclusion criteria were (a) presence of a disease such as dementia that causes volume loss in the brain or changes in blood supply and metabolism; (b) history of head trauma causing structural changes in the brain; c) history of a cerebrovascular event; (d) diagnosis of schizophrenia or other psychotic disorders; (e) epilepsy; (f) mental retardation; (g) illiteracy preventing filling out the scales; (h) history of substance or alcohol addiction; and i) pregnancy.

Assessment tools
Sociodemographic and clinical data form
This form provided the patient's sociodemographic data, the duration of the patient’s disease history, the findings of the mental status examination, and the diagnosis according to DSM-5 criteria. A semi-structured interview chart was used to record sociodemographic data, such as age, gender, active hand, education level, social support, marital status, number of children, time of first diagnosis, duration of illness, presence of psychotic symptoms, attacks, history of suicide, history of drug use, history of hospitalization, treatment, and additional drugs used, and presence of additional medical and psychiatric diseases.
Measurement of brain glucose metabolism with 18-FDG-PET/CT
All participants underwent 18-FDG-PET/ CT scans after a minimum of 6 h of fasting. The imaging made on the General Electric Discovery IQ 5 Ring device were evaluated using the IQ Cortex program on an AW 4.7 workstation. Glucose metabolism in the brain regions was recorded as a Z score and an uptake ratio. Brain regions were examined separately as the right and left lobes. Brain regions whose metabolism was recorded were the amygdala and OB.
Z score
Each PET volume was processed with 3D SSP, and uptake maps were created by calculating the average and standard deviation maps for each individual on a pixel basis. The algorithm then compared the patient's uptake map with normal brains (healthy individuals with no comorbidities) matched for age, weight, height, gender, and smoking status on a pixel basis to obtain the patient's Z score. In other words, the Z score served as an indicator of how many standard deviations the patient's FDG uptake was from a normal brain according to the patient’s age (Minoshima et al. 1995).
Quantification
The [18F]FDG activity was quantified using a fully automated post processing application, CortexID Suite 2.1 Ext. 6, a subset of the AW VolumeShare 7 software (GE Healthcare, Milwaukee, WI, USA). Each set of study data was compared to a dataset of normal controls provided through the software, with age-matched comparisons for [18F]FDG. Semiquantitative outcome measures were regional SUVR, calculated relative to a reference region, and Z scores.
Statistical analysis
Descriptive statistics of the data were given as mean and standard deviation for continuous variables and frequency and percentage for categorical variables. Normal distribution tests of numerical variables obtained from the study were performed using Kolmogorov–Smirnov and Shapiro–Wilk tests. The variables showed normal distribution (p > 0.05). An independent samples t test was used to compare the OB and amygdala metabolism (uptake) with the Z scores of the patients. Analyses were conducted using the SPSS 22.0 program. A value of p < 0.05 was considered statistically significant.
Results
In total, 12 women (46.15%) and 14 men (53.85%) participated in the study. Of the participants, 9 were married (34.62%), 17 (65.38%) were single, and the main age was 25.81 ± 4.72. The mean total disease year of the participants was 6.77 ± 4.31. The mean total number of manic episodes in the past was 3.77 ± 3.70 and the mean total number of depression episodes in the past was 3.38 ± 2.73. The maximum number of attempted suicides in the past for any patient was 8 and the mean was 0.85 ± 1.64. While 24 (92.31%) of the participants actively used their right hand, 2 (7.69%) used their left hand more actively. The sociodemographic data of the cases are shown in Table 1.
Twenty (76.92%) of our cases had psychotic symptoms at least once in the past, while 6 (23.08%) had no psychotic symptoms. While 7 (26.92%) of our participants currently used mood stabilizer (DDD) lithium, 19 of them used valproic acid. While 14 of our cases (53.85%) had received electroconvulsive therapy (ECT) in the past, 12 (46.15%) had not.
Significant differences were detected between the cases and the Z scores in terms of the OB, left OB, right amygdala, and left amygdala (Table 2).
Metabolism in the right and left amygdala was positively correlated with right OB metabolism. Metabolism in the right amygdala was correlated with the left amygdala, while the metabolism in the right and left OB were also correlated (Table 3).
Discussion
Patients with BD have been found to have a significant impairment in olfactorious identification (UPSIT) and social cognition measures compared to healthy controls (Lahera et al. 2013). A study on smell recognition in patients with BD and major depression reported that patients with BD depressive episodes and unipolar depression had a greater odor loss compared to control groups. This decreased olfactorious recognition memory persists in euthymic bipolar patients after remission of the depressive episode (Kazour et al. 2020). Lahera et al. reported that the deficiency in odor identification in patients with the euthymic period of BD was permanent and related to permanent cognitive impairment of BD patients in remission (Lahera et al. 2016). Our search of the literature revealed no previous study examining its relationship with OB in bipolar disorder. As mentioned in the introduction, although much evidence supports a decrease in the sense of smell, our interest was drawn to the increase in OB metabolism. The high metabolism in BD with intense mood alterations supports our hypothesis that the OB played a dominant role in emotional processing, rather than smell, during the evolutionary process.
The OB is one of the two structures in the brain that continues neurogenesis with the dentate gyrus of the hippocampus, receiving and integrating new neurons throughout its life (Ming and Song 2005). One important piece of information about adult neurogenesis is that it alters the structural and functional mechanisms of the brain. In our study, alterations in OB metabolism (i.e., an increase in glucose metabolism) may suggest that OB neurogenesis may be increased in BD. Of course, hypermetabolism does not directly indicate neurogenesis; nevertheless, the interpretation we can make in line with our hypothesis is as follows: recent studies have suggested that adult neurogenesis is not only a reparative system, but it is also a form of plasticity that allows the brain to adapt to the ever-evolving interaction between external stimuli and its internal response to these stimuli (Lledo and Valley 2016). The increased environmental stressors and external stimuli in BD may therefore increase OB neurogenesis. Considering the functionality of the OB, bulbar neurogenesis may be beneficial for the organism in terms of reproduction, survival, and fitness. OB neurogenesis has been shown to play a role in coupling behavior, and pheromones released by male mice increase OB neurogenesis in female mice (Mak et al. 2007). In light of this information, our findings suggest that BD may be associated with olfactorious neurogenesis in the OB.
In addition to the OB, the amygdala, orbitofrontal cortex, and hippocampus are responsible for odor identification. In particular, the amygdala and hippocampus are important parts of the limbic system involved in memory, processing emotions, and evaluating olfactory stimuli. These regions are also known to be affected in BD (Pouliot and Jones-Gotman 2008). Evidence from various functional neuroimaging studies has suggested an abnormality in amygdala activation in BD (Altshuler et al. 2005). However, structural neuroimaging studies of this brain region in patients with BD have revealed inconsistent findings.
In general, understanding the abnormalities in the structure of the amygdala will contribute to our understanding of BD pathophysiology. Similar to our results, Mah et al. found higher amygdala metabolism in patients with a BD depressive episode than in control groups in their study using 18FDG/PET (Mah et al. 2007). While our study examined patients in the euthymic period, the depressive episodes were also examined and similar results were obtained. Conversely, Al Mousawi et al. reported lower glucose metabolism in the left amygdala compared to the control group in a PET study of BD manic episodes (Spielberg et al. 2016). Drevets et al. reported increased blood flow and glucose metabolism in the amygdala and temporal lobes, along with an enlarged amygdala in BD (Drevets 2003). Blumberg et al., using PET imaging, reported increased hyperactivity in the left dorsal anterior cingulate and left head of the caudate and amygdala in manic BD (Blumberg et al. 2000). Similar to the PET studies, fMRI studies have shown increased amygdala activity in mania, depression, and BD patients in remission (Pouliot and Jones-Gotman 2008; Turetsky et al. 2012; Wesley et al. 2018; Wrynn et al. 2000).
The present study is the first to examine OB metabolism in patients with BD and to examine the relationship between OB metabolism and amygdala metabolism. We found no studies involving OB metabolism in BD in our literature review. However, the use of an olfactorious bulbectomy model of depression showed significant decreases in signal intensity in the frontal, cingulate, and occipital cortices, caudate, and amygdala (Wrynn et al. 2000). Depressive symptoms were also diminished after the regular administration of antidepressants in rodents with bulbectomy, suggesting that this would be a useful model for depression(Song and Leonard 2005). Olfactorious stimuli are one of the strongest predictors of amygdala activation, and emotional learning, a form of deterrent conditioning, has also been associated with a significant increase in activation (Costafreda et al. 2008). Our findings indicate that the increase in amygdala metabolism is associated with a higher metabolism in the OB, and this association may indicate a significant correlation between them. In BD, the main cause of hypermetabolism in the amygdala may be increased metabolism in the OB.
One of the limitations/deficiencies of our study is its small number of included patients. This study can be repeated with a larger number of patients in the future. In addition, our patients were selected only from the euthymic period, and this study can be repeated in both the depression and mania periods in future studies, allowing a comparison of all 3 periods. Another limitation is that the study was conducted while the patients were under treatment but receiving different treatments (depakine, lithium, antipsychotics, etc.). In the future, this type of study can contribute to a better understanding of patients in remission who are followed up without treatment.
Conclusion
This study showed that OB and amygdala metabolism are affected in patients with BD, suggesting that the OB, in conjunction with the amygdala, may be responsible for the etiopathogenesis of BD. The high metabolism in both the amygdala and OB in BD supports our hypothesis that the OB plays a dominant role in the emotional process.
To the best of our knowledge, our study is the first to measure the OB volume and glucose metabolism and its relationship with the amygdala in BD. The data obtained from our study contribute to the literature by providing a better understanding of the etiopathogenesis of BD and showing the effects of structural and functional changes in the OB and amygdala on BD.
References
Abler B, Greenhouse I, Ongur D et al (2008) Abnormal reward system activation in mania. Neuropsychopharmacology 33:2217–2227
Al-Mousawi AH, Evans N, Ebmeier KP et al (1996) Limbic dysfunction in schizophrenia and mania. Br J Psychiatry 169:509–516
Altshuler L, Bookheimer S, Proenza MA et al (2005) Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry 162:1211–1213
Belvederi Murri M, Ekkekakis P, Magagnoli M et al (2019) Physical exercise in major depression: reducing the mortality gap while improving clinical outcomes. Front Psychiatry 9:762
Blumberg HP, Stern E, Martinez D et al (2000) Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 48:1045–1052
Blumberg HP, Donegan NH, Sanislow CA et al (2005) Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology 183:308–313
Blumberg HP, Charney DS, Krystal JH (2002) Frontotemporal neural systems in bipolar disorder. In: Seminars in clinical neuropsychiatry. pp 243–254. https://doi.org/10.1053/scnp.2002.35220
Brewer WJ, Wood SJ, McGorry PD et al (2003) Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry 160:1790–1794
Carmichael ST, Clugnet M, Price JL (1994) Central olfactory connections in the macaque monkey. J Comp Neurol 346:403–434
Chen C, Suckling J, Lennox BR et al (2011) A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord 13:1–15
Costafreda SG, Brammer MJ, David AS, Fu CHY (2008) Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70
Croy I, Hummel T (2017) Olfaction as a marker for depression. J Neurol 264:631–638
Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237
Drevets WC (2003) Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 985:420–444
First MB (2014) Structured clinical interview for the DSM (SCID). The encyclopedia of clinical psychology 1–6
Foland LC, Altshuler LL, Bookheimer SY et al (2008) Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res Neuroimaging 162:27–37
Gottfried JA (2006) Smell: central nervous processing. In: Taste and smell. Karger Publishers, pp 44–69. https://doi.org/10.1159/000093750
Houenou J, Frommberger J, Carde S et al (2011) Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord 132:344–355
Hough CJ, Ursano RJ (2006) A guide to the genetics of psychiatric disease. Psychiatry 69:1–20
Hughes GM, Teeling EC, Higgins DG (2014) Loss of olfactory receptor function in hominin evolution. PLoS ONE 9:e84714
Jouhanneau M, Szymanski LA, Keller M (2014) Female puberty acceleration by male odour in mice: neural pathway and behavioural consequences. https://doi.org/10.1042/BST20140048
Kalmar JH, Wang F, Chepenik LG et al (2009) Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 48:636–642
Kazour F, Richa S, Char CA et al (2020) Olfactory memory in depression: state and trait differences between bipolar and unipolar disorders. Brain Sci 10:189
Lahera G, Ruiz-Murugarren S, Fernandez-Liria A et al (2013) 2078–Relationship between olfactory function and social cognition in euthymic bipolar patients. Eur Psychiatry 28:1
Lahera G, Ruiz-Murugarren S, Fernández-Liria A et al (2016) Relationship between olfactory function and social cognition in euthymic bipolar patients. CNS Spectr 21:53–59
Lawrence NS, Williams AM, Surguladze S et al (2004) Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55:578–587
Liu H, Tang Y, Womer F et al (2014) Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr Bull 40:469–477
Lledo P-M, Valley M (2016) Adult olfactory bulb neurogenesis. Cold Spring Harb Perspect Biol 8:a018945
Mah L, Zarate CA Jr, Singh J et al (2007) Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry 61:765–775
Mak GK, Enwere EK, Gregg C et al (2007) Male pheromone–stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci 10:1003–1011
McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28. https://doi.org/10.1146/annurev.neuro.27.070203.144157
Mesholam RI, Moberg PJ, Mahr RN, Doty RL (1998) Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol 55:84–90
Ming G, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250
Minoshima S, Frey KA, Koeppe RA et al (1995) A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36:1238–1248
Northcutt RG (2002) Understanding vertebrate brain evolution. Integr Comp Biol 42:743–756
Odone A, Lugo A, Amerio A et al (2020) COVID-19 lockdown impact on lifestyle habits of Italian adults. Acta Bio Medica: Atenei Parmensis 91:87
Pouliot S, Jones-Gotman M (2008) Medial temporal-lobe damage and memory for emotionally arousing odors. Neuropsychologia 46:1124–1134
Rich BA, Vinton DT, Roberson-Nay R et al (2006) Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci 103:8900–8905
Sah P, Faber ESL, Lopez de Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–834
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647
Spielberg JM, Beall EB, Hulvershorn LA et al (2016) Resting state brain network disturbances related to hypomania and depression in medication-free bipolar disorder. Neuropsychopharmacology 41:3016–3024
Szymanski LA, Keller M (2014) Activation of the olfactory system in response to male odors in female prepubertal mice. Behav Brain Res 271:30–38
Thome J, Deloyer J, Coogan AN et al (2021) The impact of the early phase of the COVID-19 pandemic on mental-health services in Europe. World J Biol Psychiatry 22:516–525
Turetsky BI, Kamath V, Calkins ME et al (2012) Olfaction and schizophrenia clinical risk status: just the facts. Schizophr Res 139:260
Wesley MS, Manjula M, Thirthalli J (2018) Interepisodic functioning in patients with bipolar disorder in remission. Indian J Psychol Med 40:52–60
Wrynn AS, Mac Sweeney CP, Franconi F et al (2000) An in-vivo magnetic resonance imaging study of the olfactory bulbectomized rat model of depression. Brain Res 879:193–199
Acknowledgements
The authors thank İ. Doğan for the assistance in statistical analysis.
Funding
This work was supported by the Gaziantep University Scientific research projects support unit that was funded by the Rectorate of Gaziantep University (2019/TF.UT.20.17).
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (MS and ŞŞ), data collection or acquisition (MS, ŞŞ, UE), statistical analysis (MS, ŞŞ), interpretation of results (MS, ŞŞ), drafting the manuscript work or revising it critically for important intellectual content (all authors) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to disclose.
Ethics approval
I verify that appropriate Institutional Review Board (IRB), and Gaziantep University ethics committee approval has been obtained with decision number 2019/515.
Consent to participate
I verify that written informed consent was obtained from all research participants.
Availability of data and material
The data are available from the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sancaktar, M., Kocamer Şahin, Ş., Demir, B. et al. Is abnormal metabolism in the olfactory bulb and amygdala associated with bipolar disorder?. J Neural Transm 130, 145–152 (2023). https://doi.org/10.1007/s00702-023-02587-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02587-9