Abstract
Studies using transcranial magnetic stimulation with simultaneous electroencephalography (TMS-EEG) revealed an imbalance between cortical excitation and inhibition (E/I) in the dorsolateral prefrontal cortex (DLPFC) in depression. As adolescence is a developmental period with an increase in depression prevalence and profound neural changes, it is crucial to study the relationship between depression and cortical excitability in adolescence. We aimed to investigate the cortical excitability of the DLPFC in adolescents with depression and a dependency of the TMS-evoked potential N100 on the depression severity. 36 clinical patients (12–18 years of age; 21 females) with a major depressive episode were assessed twice in a longitudinal design: shortly after admission (T0) and after six weeks of intervention (T1). GABA-B-mediated cortical inhibition in the left and right DLPFC, as assessed by the N100, was recorded with EEG. Significantly higher depression scores were reported at T0 compared to T1 (p < 0.001). N100 amplitudes were significantly increased (i.e., more negative) at T0 compared to T1 (p = 0.03). No significant hemispheric difference was found in the N100 component. The correlation between the difference in depression severity and the difference in N100 amplitudes (T0–T1) obtained during stimulation of the left DLPFC did not remain significant after correction for testing in both hemispheres. Higher N100 amplitudes during a state of greater depression severity are suggestive of an E/I imbalance in the DLPFC in adolescents with an acute depressive episode. The N100 reduction potentially reflects a normalization of DLPFC over inhibition in association with decreased depressive symptomatology, indicating severity dependency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Trial registration
The main longitudinal controlled add-on sports intervention study was registered with the German Clinical Trials Register (DRKS ID: DRKS00011772).
Introduction
Depression is the leading cause of disability worldwide. The associated risk of chronicity and suicidality emphasizes the need for effective treatment, in particular among youth (World Health Organization 2017). Identifying potential biomarkers of depression in adolescents is relevant in elucidating the pathophysiology of depression and enabling evaluations of treatment effects.
The dorsolateral prefrontal cortex (DLPFC) has been implicated in the neurobiology of depression, given its role in regulating negative emotions (i.e., reappraisal/suppression strategies) (Koenigs and Grafman 2009; Lévesque et al. 2003). Neuroimaging studies illustrated reduced DLPFC activity in patients with major depressive disorder (MDD) (Biver et al. 1994; Siegle et al. 2007), and the DLPFC is a prominent target for repetitive transcranial magnetic stimulation (rTMS) in the treatment of MDD (Daskalakis et al. 2008). Moreover, reduced levels of the excitatory neurotransmitter glutamate and the main inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in MDD have been reported in the cortex in general and—more specifically—the prefrontal cortex (Hasler et al. 2007; Sanacora et al. 1999; Yüksel and Öngür, 2010). Particular significance in the pathogenesis of depression is attributed to the GABAergic system in the prefrontal cortex (Duman et al. 2019; Ghosal et al. 2017; Page and Coutellier 2019) and an imbalance between cortical excitation and inhibition (E/I) has been proposed as key mechanism (Krystal et al. 2002; Lener et al. 2017). Hence, investigating the E/I balance in the DLPFC, and more specifically the role of the GABAergic system, is important to further understand the pathophysiology of MDD.
While rTMS is used to induce changes in cortical excitability (Daskalakis et al. 2008; Grossheinrich et al. 2013), single-pulse TMS combined with electroencephalography (EEG) allows for the measurement of cortical excitability. Time-logged positive and negative EEG deflections following the TMS pulse are referred to as TMS-evoked potentials (TEPs) (Tremblay et al. 2019). A frequently studied TEP is the N100—a negative deflection approximately 100 ms after the TMS pulse (Bender et al. 2005; Komssi et al. 2004; Nikulin et al. 2003). The N100 reflects GABA-B mediated inhibitory processes (Premoli et al. 2014).
In cross-sectional studies of both adults and young adults, patients with MDD exhibited greater (i.e., more negative) N100 amplitudes in the DLPFC compared with healthy participants (Dhami et al. 2020; Voineskos et al. 2019), reflecting higher DLPFC inhibition. However, these between-group designs cannot elucidate whether N100 alterations in patients with MDD reflect a temporarily and situationally stable trait or a dependency on the severity of the depressive state. Severity-dependency of the N100 in adults with MDD has been indicated in a longitudinal study by reductions in N100 amplitudes consistent with a decrease in symptom severity after a six-week rTMS-EEG intervention (Voineskos et al. 2021). Correlations between symptom severity and N100 amplitudes in adults (Voineskos et al. 2019, 2021) suggests that the more severe the depressive symptoms, the higher the N100 amplitude (i.e., more negative), further emphasizing a dependency of the N100 on depressive symptoms severity. However, N100 amplitudes did not significantly change in young adults (16–24 years of age) with MDD, during a 2-weeks theta burst therapy (Dhami et al. 2021). The absence of an association between N100 amplitudes and clinical characteristics (Dhami et al. 2021, 2020) could instead indicate a N100 trait-dependency.
Neurobiological differences associated with developmental processes might explain contradictory N100 findings between adults and young adults with MDD (Zalsman et al. 2006). An imbalance in cortical maturation between earlier developing limbic systems (associated with emotional reactivity), and later developing prefrontal cortical control systems has been proposed (Casey 2015; Casey et al. 2008). Indeed, maturation of the DLPFC was observed as late as at the end of adolescence (Gogtay et al. 2004). Hence, age dependent refinements in organization and efficiency in the DLPFC define adolescence as a sensitive period (Casey 2015; Casey et al. 2001, 2008). An association between developmental DLPFC changes and the strong increases in the prevalence of depression onset from late childhood to young adulthood has been suggested (Davey et al. 2008). Additionally, the inhibitory GABAergic system undergoes extensive changes during adolescence (Caballero and Tseng 2016) and an association between GABA-B markers and age has been observed in children/adolescents with and without depression (Croarkin et al. 2014). Therefore, it is crucial to investigate the relationship between depression and the N100 in adolescence.
To our knowledge, this is the first study to examine the TMS-EEG evoked N100 in the DLPFC as a potential biomarker for MDD in adolescence in a pre-post-intervention design. We expected a significant reduction in depressive symptom severity during a six-week inpatient intervention and either no change in N100 amplitudes (trait marker) or a significant reduction in N100 amplitudes (marker for depression severity). We hypothesized a relationship between the change in depression severity and N100 amplitudes in case a reduction in N100 amplitudes was observed.
Materials and methods
Data were collected as part of a longitudinal controlled add-on sports intervention study, in which participants received a 6-weeks sports therapy in addition to their treatment as usual. The main study was registered in the German Clinical Trials Register (DRKS- ID: DRKS00011772) and a study protocol has been published (Oberste et al. 2018). The experimental group conducted a whole-body vibrations training by performing static and dynamic exercises (e.g., squats, lunges) on the Galileo® Whole Body Vibration Plate Med M (Novotec Medical GmbH, Pforzheim, Germany). The control group executed a myofascial training without muscular or cardiovascular strain, to control for psychosocial attention, by performing seven standardized exercises (e.g., self-massage of legs, arms and back) using a foam roll (Blackroll, Bottighofen, Switzerland). Please refer to the published study protocol for more information about all assessed parameters and details of the sport intervention (Oberste et al. 2018).
At the time of the interim analysis, subjects are still being recruited as part of the clinical trial. The therapeutic effects of the intervention will be analyzed when the clinical trial is completed (N = 82) and a sufficient number of participants per exercise group has been included (n = 41 per group) according to the a priori calculated power analysis (Oberste et al. 2018). However, in the interim analysis, we were not interested in examining the therapeutic effects of the sports intervention on the primary (depressive symptoms) or secondary (neurophysiological) endpoints. Instead, we focused on the fundamental relationship between depressive symptoms and the neurophysiological parameter N100 and changes in both parameters over time. Hence, data from both intervention arms (inpatient treatment as usual plus (a) add-on control-sports intervention or (b) add-on active-sports intervention) were collapsed for baseline (T0) and post-intervention (T1) measurements. Pooling the data from both exercise groups also results in a higher number of cases (n = 36) and thus a higher statistical power, which should be sufficient for the interim analysis after about half of the planned total sample size.
Individuals and recruitment
Adolescent patients with MDD were recruited at the Clinic and Polyclinic for Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, University Hospital Cologne by psychologists and psychiatrists. 38 participants completed T0 and T1 but two (~ 5%) TMS-EEG data sets contained non-removable confounding artifacts. Therefore, 36 participants entered the analysis (21 girls; mean age 15.5 ± 1.42 years).
Detailed inclusion and exclusion criteria are reported in the study protocol (Oberste et al. 2018). Inclusion criteria were diagnosis of a current depressive episode (see “Clinical assessment”), clinical treatment and age between 13 and 18 years. In accordance with the study protocol patients with acute substance abuse or addiction, a body mass index of < 16 kg/m2, acute suicidality, pervasive developmental disorder, IQ of < 70, schizophrenia, acute psychosis, or schizoaffective disorder were excluded. Conditions limiting sportive ability, Addison's disease, untreated hypothyroidism, and long-term medication with psychotropic drugs (e.g., neuroleptics) led to exclusion from study participation. Stable antidepressant medication for a period of at least six weeks before enrollment was accepted, provided that the medication continued unchanged during the study. Two (~ 5%) individuals had a stable antidepressive medication with Fluoxetine. Due to recruitment difficulties deviations from the study protocol were made. One participant was 12 years old at enrollment but turned 13 during inpatient treatment, and two participants were allowed to sleep at home and thus became day clinic patients who nonetheless received the same treatment as usual as inpatients. The duration of study enrollment was extended so that participants were enrolled within the first four weeks of clinical treatment instead of the first three weeks, provided they still met the inclusion criteria (see “Clinical assessment”). TMS safety guidelines were considered (Rossi et al. 2011).
Clinical assessment
Individuals had a clinical diagnosis of MDD (F32.X and F33.X). ICD-10 criteria for a current depressive episode were confirmed via information assessed with the semi-structured clinical interviews Children’s Depression Rating Scale Revised (CDRS-R) (Keller et al. 2012) and the depression specific questionnaire Depressionsinventar für Kinder und Jugendliche (DIKJ) (Stiensmeier-Pelster et al. 2014) before enrollment. A raw score of ≥ 18 on the German DIKJ, which is based on the Children’s Depression Inventory (CDI) (Kovacs, 1992), further substantiated acute depressive symptoms (similar to the enrollment process in the conducted pilot study (Wunram et al. 2018)). The DIKJ corresponds to the MDD criteria of the DSM-5 (Stiensmeier-Pelster et al. 2014). Mean DIKJ score at T0 was 29.53 (SD = 7.13). Details of psychiatric assessments on axis-I comorbidity via the German version of the semi-structured clinical interview Kiddie Schedule for Affective Disorders and Schizophrenia—Present and lifetime version (K-SADS-PL) (Kaufman et al. 1997) are reported in the supplementary material (Online Resource Table A1).
Severity of depressive symptoms was assessed as primary outcome with the German version of the semi-structured clinical interview CDRS-R by blind raters. The CDRS-R follows the diagnostic criteria of the DSM-IV-TR (Keller et al. 2012) and is a common outcome measure in studies of MDD in adolescence (Plener et al. 2012; Richardson et al. 2014).
Transcranial magnetic stimulation
Using a MagPro X100 with MagOption TMS stimulator (MagVenture, Farum, Denmark), biphasic single TMS pulses were applied to the left and right DLPFC by a figure-of-eight coil (MCF-B65, outer diameter 2 × 75 mm). The TMS clicking noise was not masked by earplugs/headphones, and no foam sheet was placed between the coil and the head. The coil was held by a trained investigator. The positions of EEG electrodes F5 and F6 were used as targets for stimulation of the left and right DLPFC, respectively. Studies have shown that this localization method is sufficiently accurate (Rusjan et al. 2010). Electromyogram data of the right first dorsal interosseous muscle (FDI) were recorded. The motor hot spot for the FDI was localized (Rossini et al. 2015) and the individual resting motor threshold (RMT) was determined by the maximum likelihood procedure (Awiszus and Borckardt 2006), using the Motor Threshold Assessment Tool (version 2.0: http://www.clinicalresearcher.org/software.htm).
EEG recording
EEG signals were measured with a 64-channel BrainAmp system (BrainProducts, Munich, Germany) and recorded at a sampling rate of 5000 Hz in the Brain Vision Recorder software (BrainProducts). The TMS-compatible EEG cap (Easycap, Germany) has an equidistant montage corresponding to the extended 10–10 system and additional electrooculogram electrodes at the nasion and under both eyes. Cz was used as recording reference and impedances below 5 kΩ were ensured.
Experimental protocol
The reported DLPFC stimulation at rest was part of a TMS experiment in which two other stimulation paradigms were performed. The other stimulation paradigms consisted of single-pulse stimulation of the right DLPFC during an emotional 1-back task and a paired-pulse protocol applied at M1. In this interim analysis, we focused on GABA-B effects in the DLPFC which is the primary outcome parameter for the TMS-EEG part of our study. In addition, the signal-to-noise ratio is lower in the task paradigm due to a lower number of TMS pulses per emotional condition in the N-back task (sadness, neutral, happiness). The N100 effects in the task condition (lower signal-to-noise ratio) and in M1 (paired-pulse secondary parameters) will be analyzed when the clinical trial has been completed, and the larger sample yields higher statistical power for these analyses. Analyses of these data have not yet been performed.
During the resting condition, participants visually fixated a cross on a screen. Presentation software 18.1. (NeuroBehavioral Systems, Berkley, USA) was used for a standardized application of TMS pulses. Forty-five TMS single pulses were applied in blocks to the left and right DLPFC in counterbalanced order with randomized stimulus intervals (5–8 s). Good signal-to-noise ratios (SNR) have been obtained for N100 peaks with ~ 45 and ~ 48 trials in previous TMS-EEG studies (Chung et al. 2017, 2018). In combination with a suprathreshold stimulation intensity of 120% RMT, a good SNR can be expected with 45 trials, especially in children and adolescents, as they show high N100 amplitudes (Bender et al. 2005).
Data analysis
Signal pre-processing
BrainVision Analyzer (BrainProducts, Munich, Germany) was used to analyze EEG data similar to previously published procedures (Roos et al. 2021). The large file size was reduced by downsampling to 500 Hz. The downsampling process with the implemented anti-aliasing filter (low-pass-filter 225 Hz) may slightly distort the TMS pulse artifact (Rogasch et al. 2017), but we verified that the examined time window (80–140 ms) was not affected. EEG data were interpolated − 10 to 20 ms around the TMS pulse to remove the TMS pulse artifact. Data were referenced to an average reference and EEG data were segmented into epochs of − 500 to 500 ms around the TMS pulse. Severe noise (e.g., large muscular artifacts) was manually rejected in individual channels and data were baseline corrected (− 110 to − 10 ms). Blink and eye movement artifacts were removed by independent component analysis (Ilmoniemi et al. 2015). Linear DC detrending and a 50 Hz notch filter were applied. An inspection revealed that the DC detrend did not change grand averages systematically but reduced variance. Averages were calculated for left and right DLPFC stimulation condition for each measurement (T0/T1).
N100 analysis
The TMS-evoked N100 maximum has been described ipsilaterally over the stimulation site (Bonato et al. 2006; Jarczok et al. 2021). Clearly lateralized ipsilateral TEPs at the stimulation site likely reflect genuine cortical activity and not merely activity due to peripheral stimulation (Conde et al. 2019). Therefore, ipsilateral electrodes F5 and F6 were selected as electrodes of interest for the left and right DLPFC stimulation condition, respectively (Ilmoniemi and Kičić 2010). Similar to previous studies, the N100 was defined as the highest negative peak in the 80–140 ms time interval at the electrode of interest (Kerwin et al. 2018; Rogasch et al. 2014, 2015). Mean amplitudes ± 10 ms around the peak were exported.
To systematically examine lateralized activity, i.e., transcranially evoked cortical activity at the stimulation site that depends on the side of stimulation (left/right), a calculation analogous to the lateralized readiness potential (LRP) (Coles, 1989) was performed. This procedure integrates information from both ipsilateral and contralateral homologous electrodes for each stimulation condition, eliminating symmetric activity that is not specific to the stimulation condition. Similar to a previous study in our group (Jarczok et al. 2021), a single measure called LatTEP N100 was calculated based on the TEPs of the homologous electrodes. The calculation is performed according to the following example procedure (Coles, 1989): LatTEP N100 F5/F6 = [F5(TMS left) − F6(TMS left) + F6(TMS right) − F5(TMS right)]/2. This formula was applied to each homologous electrode pair, resulting in a topographic plot on one side for each time point, which contains information on the lateralized activity of both stimulation conditions (left/right). The peak for the LatTEP N100 component was placed in the 80–140 ms time window in the reference channel F5/F6, and mean amplitudes ± 10 ms around the peak were exported.
Statistics
IBM SPSS Statistics 28 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Shapiro-Wilks tests revealed a normal distribution of CDRS-R values at both time points and a normal distribution of CDRS-R and N100 amplitude difference values (T0–T1). The normality assumption was neither met for all N100 amplitudes in each stimulation condition (time point × stimulation site), LatTEP N100 F5/F6 amplitudes at T0 and T1 nor for age. The N100 amplitudes of each stimulation condition were nonetheless entered into a two-way repeated-measures analysis of variance (rANOVA) because it is robust against violations of normality and, as a parametric method, has the advantage of higher statistical power compared with nonparametric methods (Blanca et al. 2017; Schmider et al. 2010). Nonparametric methods were used for comparisons between LatTEP N100 F5/F6 amplitudes at T0 and T1 as well as correlational analysis between N100 amplitudes of each stimulation condition and age and sex, due to the violation of normality.
Outlier analysis revealed two extreme outliers of more than ± 3 standard deviations (SD) from the mean (M) N100 amplitude during stimulation of the left DLPFC at T0. In this condition, 90% winsorization was performed by replacing the upper and lower 5 percent of the N100 amplitudes with the value of the 5th and 95th percentiles, respectively, to avoid distortions by outliers (Leys et al. 2019; Tukey and McLaughlin 1963).
Reductions in depressive symptom severity, as assessed by CDRS-R scores at T0 and T1, were examined by a dependent t-test. Two-way rANOVAs with TIME (T0/T1) and STIMULATION SIDE (left stimulation at electrode F5/right stimulation at electrode F6) as within-subject factors were used with the dependent variable N100 amplitudes to test our hypothesis on N100 amplitude alterations. A nonparametric Wilcoxon signed-rank test was applied to test the difference in LatTEP N100 amplitudes at F5/F6 between T0 and T1. To estimate the effect size, the z-score was normalized by sample size, i.e., r = z/\(\sqrt{N}\) (Rosenthal 1991).
Two correlational analyses were performed: Spearman’s correlation coefficients were calculated between N100 amplitudes and age as well as sex, because the N100 amplitudes in each stimulation condition (time point × stimulation site) were not distributed normally. Since the N100 and CDRS-R difference values were distributed normally, parametric Pearson correlations between CDRS-R difference values and N100 amplitudes difference values (T0–T1) were evaluated by one-sided tests according to the hypothesis. For multiple comparisons, p-values were Bonferroni-Holm corrected and a statistical significance level of p < 0.05 was applied.
Results
Depressive symptoms
A dependent t-test demonstrated a signficiant decrease in depressive symptom severity by higher CDRS-R scores at T1 (M = 44.83, SD = 13.15) compared with T0 (M = 54.97, SD = 13.71) (difference: M = 10.14, SD = 11.36; t(35) = 5.35, p < 0.001, d = 0.89).
N100 amplitudes and difference values
A rANOVA with the dependent variable N100 amplitude returned a significant main effect for the within-subject factor TIME (Table 1), with larger N100 amplitudes at T0 measurement (Figs. 1 and 2).
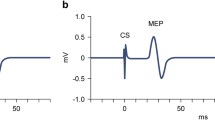
Group grand average TEP amplitudes and N100 topographies. Butterfly plots (a) are illustrated for each stimulation condition. Red lines indicate the TEP time course at electrode F5 during left DLPFC stimulation (a1 and a3) and at F6 during right DLPFC stimulation (a2 and a4). TEP time courses for each time point (T0, T1) as well as difference waves (T0–T1) are displayed for the left (b1) and right (b2) stimulation condition at the respective electrode of interest (F5 and F6). Dark blue and dark red lines (squared arrowheads) indicate amplitudes measured at baseline (T0). Lighter blue and lighter red lines (round arrowhead) indicate amplitudes measured post-intervention (T1). Dotted lines indicate the difference wave between T0 and T1. Light gray bars highlight the time window (80–140 ms) used for the N100 peak detection. The TMS artifact (dark gray bars) has been cut out. The topographical maps illustrate the activity during left (b3–b5) and right (b6–b8) stimulation at T0 and T1 as well as the difference between T0 and T1. To illustrate N100 topographies the time window 120–130 ms was chosen, based on peaks in the grand average. Variations in individual peak width explain earlier mean peak latencies based on single subject averages compared with peak latencies displayed in grand averages. Lateralized activity maps for T0 (c1) and T1 (c2) illustrate TMS‐evoked cortical activation, in which symmetrical activity between hemispheres is subtracted. As a result of the calculation between homologous electrodes, one channel remains (depicted arbitrarily on the left side of the head), which encompasses signals from both stimulation sites (left and right stimulation) and both hemispheres. Yellow circles mark the position of electrodes of interest (F5/F6) in the topographies. Please note that the original data maps are plotted on 5 μV scale while lateralized activity was plotted on 2 μV scale
Error bar chart of the mean N100 amplitudes at the ipsilateral electrode of interest during left (F5) and right (F6) DLPFC stimulation at baseline (T0) and post-intervention measure (T1). Error bars reflect 95% confidence intervals adapted to repeated-measures designs (Cousineau 2005)
Descriptive statistics on N100 amplitudes and latencies are provided in Table 2. No significant correlations between N100 amplitudes (scores at T0, T1 and difference values) and age or sex were found (see Online Resource Table B1).
LatTEP N100 F5/F6 amplitudes
The Wilcoxon signed-rank test revealed a trend toward significantly higher LatTEP N100 amplitudes at electrode F5/F6 during the T0 measurement compared with the T1 measurement (Z = − 1.82, p = 0.07, r = − 0.30). Descriptive statistics on LatTEP N100 F5/F6 amplitudes and latencies are provided in Table 2.
Association between depressive symptoms and N100
Pearson correlations between CDRS-R difference values and N100 amplitude difference values (T0-T1) were significant for the left DLPFC (F5) (r = − 0.30, p = 0.04, one-tailed), but not for the right DLPFC stimulation condition (F6) (r = − 0.12, p = 0.25, one-tailed). After correction for two tests on the two hemispheres, a trend towards significance for the left stimulation condition remained (r = − 0.30, p = 0.07, one-tailed).
Discussion
Examining N100 alterations longitudinally in adolescents with depression, we found significantly increased (i.e., more negative) N100 amplitudes in the DLPFC at baseline, coinciding with significantly more severe depressive symptoms. Thus, N100 amplitudes seem to be dependent on the depressive symptom severity in adolescents.
N100 amplitudes and depression
Our results indicate altered cortical inhibition in the DLPFC depending on the depressive symptom severity in adolescents, as the N100 amplitude is considered to reflect GABA-B mediated cortical inhibition (Premoli et al. 2014; Rogasch et al. 2015). Higher N100 amplitude in a state with greater depression severity is congruent with cross-sectional greater N100 amplitudes in the DLPFC in (young) adults with MDD compared to healthy controls (Dhami et al. 2020; Voineskos et al. 2019). Longitudinal N100 amplitude decreases in association with an improvement in symptom severity have also been observed in adults, receiving six-week active rTMS stimulation (Voineskos et al. 2021). A trend towards a significant moderate correlation between reductions in N100 amplitude and depressive symptom severity in our data could indicate a clinically relevant association. The fact that the correlation was no longer significant after correction for multiple testing could be due to the large variability in difference values (T0–T1) between subjects, and the relationship might become more distinct in a larger sample.
Contrary, a lack of significant alterations in N100 amplitudes in young adults (16–24 years, n = 16) with MDD during a two-weeks theta burst intervention (Dhami et al. 2021) might argue against a dependency of N100 amplitudes on depression severity. Even though the N100 amplitude reductions were not significant, arguably due to the smaller sample size, descriptively larger N100 amplitudes at baseline were illustrated. That N100 changes reflect only order effects due to multiple measurements (T0 and T1) and not systematic changes as a function of depressive symptom severity seems unlikely, as TEPs are sensitive to changes in cortical properties as well as repeatable over time (Casarotto et al. 2010) and the N100 shows good reliability (Kerwin et al. 2018; Lioumis et al. 2009).
The found alterations in N100 amplitudes, indicating increased cortical inhibition, can be related to changes in synaptic transmission during a depressive episode. Increased prefrontal inhibition during a depressive episode has been explained in previous studies by an increased GABA turnover postsynaptically (Dhami et al. 2020; Voineskos et al. 2019) and/or an E/I imbalance, in which inhibition still outweighs excitation (Page and Coutellier 2019). Depression related deficits in GABA neurotransmitter levels (Duman et al. 2019; Hasler et al. 2007) might be over-compensated by increased activity of specific inhibitory GABAergic interneurons (Page and Coutellier 2019). The corresponding elevated interneuron activity might lead to increased inhibition and subsequently a hypoactivity of the prefrontal cortex, thus causing a higher TMS-EEG inhibitory N100 marker (Page and Coutellier 2019). This proposed aberrant E/I balance likely depends on the depression severity, as antidepressant effects of N-methyl-d-aspartate antagonist ketamine is caused by extensive glutamate release (Abdallah et al. 2018; Krystal et al. 2013; Lener et al. 2017) and other antidepressant therapies lead to increased cortical GABA levels during treatment (Bhagwagar et al. 2004; Dubin et al. 2016; Sanacora et al. 2003). N100 amplitude reductions during an antidepressant treatment therefore likely reflect ‘normalization’ of the E/I balance. The ‘normalization’ of the E/I imbalance and the antidepressant effect of our therapy could be due to an increase in GABA concentration, similar to other antidepressant therapies (Bhagwagar et al. 2004; Dubin et al. 2016; Sanacora et al. 2003). The normalization of the GABA concentration could in turn reverse the proposed over-compensatory activity of GABA-B interneurons. Thus, the reduced activity of GABA-B interneurons could lead to reduced hypoactivity in the DLPFC when the severity of depressive symptoms decreases. The reduced activity of GABA-B interneurons might be reflected in smaller N100 amplitudes after the intervention.
Lateralized N100 amplitudes
To further investigate the reduction in N100 amplitudes between T0 and T1, the systematically lateralized activity to the stimulation side (left/right) was considered in more detail. TEP amplitudes have been shown to be systematically higher ipsilaterally than contralaterally, with the highest amplitudes at the stimulation site (Jarczok et al. 2021). A lateralized ipsilateral topography around the stimulation site is likely not only the result of peripheral stimulation (Conde et al. 2019) but rather transcranially evoked. The topographic maps of the original data (Fig. 1b) confirm a lateralized ipsilateral maximum at each stimulation site. To eliminate evoked activity that is not systematically lateralized at the stimulation site, we calculated LatTEP N100 amplitudes at the homologous electrodes of interest F5/F6. This process can reveal lateralized negativity at the site of stimulation that could be masked by symmetric processes in the original maps. The topographic maps of the LatTEP (Fig. 1c) show a negative maximum at the stimulation site (F5/F6) for T0 and T1 in the same time window as the original data (120–130 ms). Although the topographies indicate lower activity at T1 compared with T0, which is consistent with the amplitude reduction in the original N100 data, the LatTEP N100 reduction at F5/F6 did not surpass the threshold of statistical significance. The trend result could be due to the slightly smaller effect of depression severity on the lateralized part of N100 compared to the effect on the overall N100 (including lateralized and non-lateralized parts of the N100). Although the trend supports our findings on the original N100 alterations between time points, a larger sample is needed to further investigate the trend in lateralized potentials.
N100 amplitudes and age
Exploring cortical inhibitory processes as a function of depressive symptom severity in various age groups seems crucial, as the prefrontal E/I balance in adolescents undergoes extensive maturational changes (Caballero and Tseng 2016; Kilb 2012; Page and Coutellier 2019). However, in this study no correlation between age and N100 amplitudes in the examined age-range was detected. Previous studies on cortical inhibition and age mainly focused on the motor cortex and reported age effects on N100 amplitudes in healthy participants of varying ages (Bender et al. 2005; Määttä et al. 2017). In 9–17-year-olds with depression a negative correlation between age and long-interval cortical inhibition (LICI), a GABA-B marker assessed by a double-pulse TMS-EEG paradigm, has also been reported (Croarkin et al. 2014). Age effects on GABA-B marker are probably driven by inclusion of pre-adolescent children as age effects were apparent when children were compared with adults and adolescents, but not when comparing adolescents to adults (Määttä et al. 2017). The lack of association between N100 and age in the investigated age group (12–18 years) is likely related to the small age variance in the analyzed sample.
Hemispheric asymmetries
We found variations between hemispheres, as a trend towards significant correlation between difference values of N100 amplitudes and symptom severity was observed only during left DLPFC stimulation. For the left DLPFC, previous studies have reported correlations between baseline N100 amplitudes and changes in suicidal ideation (Sun et al. 2016) and correlations between N100 amplitudes and depressive symptoms (Voineskos et al. 2021, 2019).
Theories on anterior hemispheric asymmetries in depression have been discussed before, as EEG (Davidson et al. 2002; Miller et al. 2013) and fMRI (Grimm et al. 2008; Herrington et al. 2010) studies suggest reduced frontal activity in the left compared with the right hemisphere. Additionally, patients with MDD benefit from therapeutic high-frequency rTMS over the left DLPFC (increasing cortical activity) and low-frequency rTMS over the right DLPFC (suppressing cortical activity) (Chen et al. 2013; Gershon et al. 2003). Frontal left/right lateralization has been linked to positive/negative emotional valence processes (Davidson 1992; Heller et al. 1998) or approach/avoidance motivation (Davidson et al. 2002). The DLPFC seems to be involved in the cognitive regulation of emotional processing in the amygdala. Hypoactivity of the left DLPFC during a depressive episode might be associated with impaired downregulation of emotions with negative valence and impaired upregulation of positive emotions (Bruder et al. 2017). Normalization of over inhibition in the left DLPFC might represent a relevant antidepressant mechanism. However, we did not observe significant differences in N100 amplitudes between hemispheres, which would be expected if the left DLPFC is less active compared with the right DLPFC. Therefore, underlying mechanism and directionality need to be investigated in future studies to examine hemispheric differences in N100 amplitudes.
Limitations
One limitation is that we did not use auditory masking. The TMS coil click results in an AEP reflected by a negative deflection at 100 ms and a positive peak 180–200 ms after the pulse (ter Braack et al. 2015). Thus, the measured N100 amplitude in our study reflects not only transcranially evoked cortical activity due to the TMS pulse but also, to some extent, peripherally evoked AEPs. However, it is important to consider the topography of the AEP-related N100 component, which has been described in central regions with bilateral distribution (Rogasch et al. 2014) or in the contralateral hemisphere during monaural acoustic stimulation (Hine and Debener 2007). A comparison between active and sham TMS stimulation conditions indicated that a N100 component with a topography over the vertex represents to a great extent a non-transcranial sensory evoked potential (Conde et al. 2019). As described previously, the lateralized ipsilateral topography of the N100 at the stimulation site (see Fig. 1) supports the notion that although the recorded N100 is affected by peripherally evoked AEPs to some extent, its ipsilateral amplitude reflects mainly transcranially evoked activity.
Despite an influence of the AEPs on the TEP N100, we believe that our results nevertheless allow valid conclusions to be drawn about intraindividual N100 changes, because stable AEP N100 components between measurements were described in intraindividual designs (Atcherson et al. 2006; Louzã et al. 1994). Moreover, the AEP N100 component seems to not be related systematically to depressive symptoms, as neither AEPs nor loudness dependency of AEPs differed between participants with and without MDD (Bruder et al. 1995; Feldmann et al. 2018; Park et al. 2010). The loudness dependent AEP at baseline measure predicts the response to antidepressive medication in patients with MDD, but the component does not change in longitudinal designs (Gallinat et al. 2000; Juckel et al. 2007; Lee et al. 2015). Although AEPs have a stable effect on N100 amplitudes, the measured N100 alterations are likely due to genuine depression related changes in the GABA-B system and not to AEP alterations.
Furthermore, TMS was perfomed without neuronavigation. Neuronavigation, although helpful during TMS coil positioning, significantly increases measurement time (Julkunen et al. 2009). Instead, coil positions were chosen based on the DLPFC localization method of Rusjan and colleagues (2010), which has been used previously in other studies (Cash et al. 2017; Fitzgerald et al. 2009; Noda et al. 2021; Rogasch et al. 2015; Sun et al. 2016).
Conclusion
In conclusion, greater N100 amplitudes during a state of greater depression severity support the hypothesis of E/I imbalance in the DLPFC in adolescents, with over inhibition normalizing with decreasing depressive symptom severity. To our knowledge, the depressive symptom severity dependency of this inhibitory marker has been demonstrated for the first time in adolescents with MDD and may enable the future use of N100 amplitude as biomarker in the context of diagnostic and therapeutic assessments.
Data availability
The ethics committee did not grant permission to share study data with third parties or to upload data in anonymized form.
References
Abdallah CG, De FHM, Averill LA, Jiang L, Averill CL, Chowdhury GMI et al (2018) The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43:2154–2160. https://doi.org/10.1038/s41386-018-0136-3
Atcherson SR, Gould HJ, Pousson MA, Prout TM (2006) Long-term stability of N1 sources using low-resolution electromagnetic tomography. Brain Topogr 19:11–20. https://doi.org/10.1007/s10548-006-0008-8
Awiszus F, Borckardt JJ (2006) TMS Motor Threshold Assessment Tool (MTAT 2.0)
Bender S, Basseler K, Sebastian I, Resch F, Kammer T, Oelkers-Ax R et al (2005) Transcranial magnetic stimulation evokes giant inhibitory potentials in children. Ann Neurol 58:58–67. https://doi.org/10.1002/ana.20521
Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews P, Cowen PJ (2004) Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry 161:368–370
Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P et al (1994) Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry 36:381–388. https://doi.org/10.1016/0006-3223(94)91213-0
Blanca MJ, Alarcón R, Arnau J, Bono R, Bendayan R (2017) Non-normal data: is ANOVA still a valid option ? Psicothema 29:552–557. https://doi.org/10.7334/psicothema2016.383
Bonato C, Miniussi C, Rossini PM (2006) Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol 117:1699–1707. https://doi.org/10.1016/j.clinph.2006.05.006
Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M et al (1995) Brain event-related potentials to complex tones in depressed patients: relations to perceptual asymmetry and clinical features. Psychophysiology 32:373–381
Bruder GE, Stewart JW, McGrath PJ (2017) Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci Biobehav Rev 78:178–191. https://doi.org/10.1016/j.neubiorev.2017.04.021
Caballero A, Tseng KY (2016) GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci 39:441–448. https://doi.org/10.1016/j.tins.2016.04.010
Casarotto S, Lauro LJR, Bellina V, Casali AG, Rosanova M, Pigorini A et al (2010) EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS ONE 5:e10281. https://doi.org/10.1371/journal.pone.0010281
Casey BJ (2015) Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol 66:295–319. https://doi.org/10.1146/annurev-psych-010814-015156
Casey BJ, Durston S, Fossella JA (2001) Evidence for a mechanistic model of cognitive control. Clin Neurosci Res 1:267–282
Casey BJ, Jones RM, Hare TA (2008) The adolescent brain. Ann N Y Acad Sci 1124:111–126. https://doi.org/10.1097/wnn.0b013e318294860b
Cash RFH, Noda Y, Zomorrodi R, Radhu N, Farzan F, Rajji TK et al (2017) Characterization of glutamatergic and GABA-A -mediated neurotransmission in motor and dorsolateral prefrontal cortex using paired-pulse TMS–EEG. Neuropsychopharmacology 42:502–511. https://doi.org/10.1038/npp.2016.133
Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y et al (2013) Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res 210:1260–1264. https://doi.org/10.1016/j.psychres.2013.09.007
Chung SW, Lewis BP, Rogasch NC, Saeki T, Thomson RH, Hoy KE et al (2017) Demonstration of short-term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: a TMS-EEG study. Clin Neurophysiol 128:1117–1126. https://doi.org/10.1016/j.clinph.2017.04.005
Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB (2018) The effect of single and repeated prefrontal intermittent theta burst stimulation on cortical reactivity and working memory. Brain Stimul 11:566–574. https://doi.org/10.1016/j.brs.2018.01.002
Coles MGH (1989) Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology 26:251–269. https://doi.org/10.1111/j.1469-8986.1989.tb01916.x
Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A et al (2019) The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage 185:300–312. https://doi.org/10.1016/j.neuroimage.2018.10.052
Croarkin PE, Nakonezny PA, Lewis CP, Zaccariello MJ, Huxsahl JE, Husain MM et al (2014) Developmental aspects of cortical excitability and inhibition in depressed and healthy youth: an exploratory study. Front Hum Neurosci 8:1–9. https://doi.org/10.3389/fnhum.2014.00669
Cousineau D (2005) Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutor Quant Methods Psychol 1:42–5.
Daskalakis ZJ, Levinson AJ, Fitzgerald PB (2008) Repetitive transcranial magnetic stimulation for major depressive disorder: a review. Can J Psychiatry 53:555–566
Davey CG, Yücel M, Allen NB (2008) The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev 32:1–19. https://doi.org/10.1016/j.neubiorev.2007.04.016
Davidson RJ (1992) Anterior cerebral asymmetry and the nature of emotion. Brain Cogn 20:125–151
Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD et al (2002) Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 52:478–502
Dhami P, Atluri S, Lee JC, Knyahnytska Y, Croarkin PE, Blumberger DM et al (2020) Prefrontal cortical reactivity and connectivity markers distinguish youth depression from healthy youth. Cereb Cortex 30:3884–3894. https://doi.org/10.1093/CERCOR/BHAA004
Dhami P, Atluri S, Lee J, Knyahnytska Y, Croarkin PE, Blumberger DM et al (2021) Neurophysiological markers of response to theta burst stimulation in youth depression. Depress Anxiety 38:172–184. https://doi.org/10.1002/da.23100
Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KAB, Kang G et al (2016) Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci 41:37–45. https://doi.org/10.1503/jpn.150223
Duman RS, Sanacora G, Krystal JH (2019) Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102:75–90. https://doi.org/10.1016/j.neuron.2019.03.013
Feldmann L, Piechaczek CE, Pehl V, Bartling J, Bakos S, Schulte-Körne G et al (2018) State or trait? Auditory event-related potentials in adolescents with current and remitted major depression. Neuropsychologia 113:95–103. https://doi.org/10.1016/j.neuropsychologia.2018.03.035
Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ (2009) Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul 2:234–237. https://doi.org/10.1016/j.brs.2009.03.002
Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss H-J et al (2000) The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology 148:404–411
Gershon AA, Dannon PN, Grunhaus L (2003) Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 160:835–845. https://doi.org/10.1097/01.PSYPHR.0000427461.48450.93
Ghosal S, Hare B, Duman RS (2017) Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci 14:1–8. https://doi.org/10.1016/j.cobeha.2016.09.012
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC et al (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci 101:8174–8179. https://doi.org/10.1073/pnas.0402680101
Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F et al (2008) Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry 63:369–376. https://doi.org/10.1016/j.biopsych.2007.05.033
Grossheinrich N, Reinl M, Pogarell O, Karch S, Mulert C, Brueckl M et al (2013) Effects of low frequency prefrontal repetitive transcranial magnetic stimulation on the N2 amplitude in a GoNogo task. PLoS ONE 8:e67136. https://doi.org/10.1371/journal.pone.0067136
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007) Reduced prefrontal glutamate/glutamine and ɣ -aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200
Heller W, Nitschke JB, Miller GA (1998) Lateralization in emotion and emotional disorders. Curr Dir Psychol Sci 7:26–32
Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG et al (2010) Localization of asymmetric brain function in emotion and depression. Psychophysiology 47:442–454. https://doi.org/10.1111/j.1469-8986.2009.00958.x
Hine J, Debener S (2007) Late auditory evoked potentials asymmetry revisited. Clin Neurophysiol 118:1274–1285. https://doi.org/10.1016/j.clinph.2007.03.012
Ilmoniemi RJ, Kičić D (2010) Methodology for combined TMS and EEG. Brain Topogr 22:233–248. https://doi.org/10.1007/s10548-009-0123-4
Ilmoniemi RJ, Hernandez-Pavon JC, Mäkelä NN, Metsomaa J, Mutanen TP, Stenroos M, et al (2015) Dealing with artifacts in TMS-evoked EEG. 37th Annu Int Conf IEEE Eng Med Biol Soc. https://doi.org/10.1109/EMBC.2015.7318342
Jarczok TA, Roebruck F, Pokorny L, Biermann L, Roessner V, Klein C et al (2021) Single-pulse TMS to the temporo-occipital and dorsolateral prefrontal cortex evokes lateralized long latency EEG responses at the stimulation site. Front Neurosci. https://doi.org/10.3389/fnins.2021.616667
Juckel G, Pogarell O, Augustin H, Mulert C, Müller-Siechender F, Frodl T et al (2007) Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J Clin Psychiatry 68:1206–1212
Julkunen P, Säisänen L, Danner N, Niskanen E, Hukkanen T, Mervaala E et al (2009) Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44:790–795. https://doi.org/10.1016/j.neuroimage.2008.09.040
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. https://doi.org/10.1097/00004583-199707000-00021
Keller F, Grieb J, Kölch M, Spröber N (2012) Children’s depression rating scale—revised by E. O. Poznanski and H. B. Mokros—Deutsche Version. 1. Hogrefe
Kerwin LJ, Keller CJ, Wu W, Narayan M, Etkin A (2018) Test-retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimul 11:536–544. https://doi.org/10.1016/j.brs.2017.12.010
Kilb W (2012) Development of the GABAergic system from birth to adolescence. Neurosci 18:613–630. https://doi.org/10.1177/1073858411422114
Koenigs M, Grafman J (2009) The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201:239–243. https://doi.org/10.1016/j.bbr.2009.03.004
Komssi S, Kähkönen S, Ilmoniemi RJ (2004) The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp 21:154–164. https://doi.org/10.1002/hbm.10159
Kovacs M (1992) Children’s depression inventory (CDI). Multi-Health Systems Inc., New York
Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G et al (2002) Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 7:71–80. https://doi.org/10.1038/sj.mp.4001021
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141. https://doi.org/10.1016/j.biopsych.2013.03.026
Lee B, Park Y, Lee S, Shim M (2015) Prediction of long-term treatment response to selective serotonin reuptake inhibitors (SSRIs) using scalp and source loudness dependence of auditory evoked potentials (LDAEP) analysis in patients with major depressive disorder. Int J Mol Sci 16:6251–6265. https://doi.org/10.3390/ijms16036251
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC et al (2017) Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 81:886–897. https://doi.org/10.1016/j.biopsych.2016.05.005
Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G et al (2003) Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53:502–510. https://doi.org/10.1016/S0002-3223(03)01817-6
Leys C, Delacre M, Mora YL, Lakens D, Ley C (2019) How to classify, detect, and manage univariate and multivariate outliers, with emphasis on pre-registration. Int Rev Soc Psychol 32:1–10. https://doi.org/10.5334/irsp.289
Lioumis P, Kičić D, Savolainen P, Mäkelä JP, Kähkönen S (2009) Reproducibility of TMS—evoked EEG responses. Hum Brain Mapp 30:1387–1396. https://doi.org/10.1002/hbm.20608
Louzã MR, Adler G, Gattaz WF (1994) Temporal stability of auditory evoked potentials at different stimulation rates. Braz J Med Biol Res 27:2413–2421
Määttä S, Könönen M, Kallioniemi E, Lakka T, Lintu N, Lindi V et al (2017) Development of cortical motor circuits between childhood and adulthood: a navigated TMS-HdEEG study. Hum Brain Mapp 38:2599–2615. https://doi.org/10.1002/hbm.23545
Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W (2013) Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci 7:1–9. https://doi.org/10.3389/fnint.2013.00002
Nikulin VV, Kičić D, Kähkönen S, Ilmoniemi RJ (2003) Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. Eur J Neurosci 18:1206–1212. https://doi.org/10.1046/j.1460-9568.2003.02858.x
Noda Y, Barr MS, Zomorrodi R, Cash RFH, Lioumis P, Chen R et al (2021) Single-pulse transcranial magnetic stimulation-evoked potential amplitudes and latencies in the motor and dorsolateral prefrontal cortex among young, older healthy participants, and schizophrenia patients. J Pers Med. https://doi.org/10.3390/jpm11010054
Oberste M, Großheinrich N, Wunram H-L, Graf JL, Ziemendorff A, Meinhardt A et al (2018) Effects of a 6-week, whole-body vibration strength-training on depression symptoms, endocrinological and neurobiological parameters in adolescent inpatients experiencing a major depressive episode (the “Balancing Vibrations Study”): study protocol for a randomized placebo-controlled trial. Trials 19:1–11. https://doi.org/10.1186/s13063-018-2747-8
Page CE, Coutellier L (2019) Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev 105:39–51. https://doi.org/10.1016/j.neubiorev.2019.07.024
Park YM, Lee SH, Kim S, Bae SM (2010) The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Prog Neuro-Psychopharmacol Biol Psychiatry 34:313–316. https://doi.org/10.1016/j.pnpbp.2009.12.004
Plener PL, Grieb J, Spröber N, Straub J, Schneider A, Keller F et al (2012) Convergence of children’s depression rating scale-revised scores and clinical diagnosis in rating adolescent depressive symptomatology. Ment Illn 4:29–31. https://doi.org/10.4081/mi.2012.e7
Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C et al (2014) TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 34:5603–5612. https://doi.org/10.1523/jneurosci.5089-13.2014
Richardson LP, Ludman E, McCauley E, Lindenbaum J, Larison C, Zhou C et al (2014) collaborative care for adolescents with depression in primary care: a randomized clinical trial. JAMA 312:809–816. https://doi.org/10.1001/jama.2014.9259.Collaborative
Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC et al (2014) Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage 101:425–439. https://doi.org/10.1016/j.neuroimage.2014.07.037
Rogasch NC, Daskalakis ZJ, Fitzgerald PB (2015) Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: a TMS-EEG study. Cortex 64:68–77. https://doi.org/10.1016/j.cortex.2014.10.003
Rogasch NC, Sullivan C, Thomson RH, Rose NS, Bailey NW, Fitzgerald PB et al (2017) Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software. Neuroimage 147:934–951. https://doi.org/10.1016/j.neuroimage.2016.10.031
Roos D, Biermann L, Jarczok TA, Bender S (2021) Local differences in cortical excitability—a systematic mapping study of the TMS-evoked N100 component. Front Neurosci. https://doi.org/10.3389/fnins.2021.623692
Rosenthal R (1991) Meta-analytic procedures for social research, 2nd edn. Sage, Newbury Park
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686. https://doi.org/10.1016/j.clinph.2010.12.037
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107. https://doi.org/10.1016/j.clinph.2015.02.001
Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB et al (2010) Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31:1643–1652. https://doi.org/10.1002/hbm.20964
Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OAC et al (1999) Reduced cortical ɣ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56:1043–1047
Sanacora G, Mason G, Rothman D, Hyder F, Ciarcia JJ, Ostroff RB et al (2003) Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160:577–579. https://doi.org/10.1176/appi.ajp.160.3.577
Schmider E, Ziegler M, Danay E, Beyer L, Bühner M (2010) Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology 6:147–151. https://doi.org/10.1027/1614-2241/a000016
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME (2007) Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 61:198–209. https://doi.org/10.1016/j.biopsych.2006.05.048
Stiensmeier-Pelster J, Braune-Krickau M, Schürmann M, Duda K (2014) Depressionsinventar für Kinder und Jugendliche—3., überarbeitete und neu normierte Auflage. 3. Hogrefe
Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS et al (2016) Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression. JAMA Psychiat 73:337–345. https://doi.org/10.1001/jamapsychiatry.2015.3097
ter Braack EM, de Vos CC, van Putten MJAM (2015) Masking the auditory evoked potential in TMS–EEG: a comparison of various methods. Brain Topogr 28:520–528. https://doi.org/10.1007/s10548-013-0312-z
Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R et al (2019) Clinical neurophysiology clinical utility and prospective of TMS–EEG. Clin Neurophysiol 130:802–844. https://doi.org/10.1016/j.clinph.2019.01.001
Tukey JW, McLaughlin DH (1963) Less vulnerable confidence and significance procedures for location based on a single sample: trimming/winsorization. Sankhyã Indian J Stat Ser A 25:331–352
Voineskos D, Blumberger DM, Zomorrodi R, Rogasch NC, Farzan F, Foussias G et al (2019) Altered transcranial magnetic stimulation-electroencephalographic markers of inhibition and excitation in the dorsolateral prefrontal cortex in major depressive disorder. Biol Psychiatry 85:477–486. https://doi.org/10.1016/j.biopsych.2018.09.032
Voineskos D, Blumberger DM, Rogasch NC, Zomorrodi R, Farzan F, Foussias G et al (2021) Neurophysiological effects of repetitive transcranial magnetic stimulation (rTMS) in treatment resistant depression. Clin Neurophysiol 132:2306–2316. https://doi.org/10.1016/j.clinph.2021.05.008
World Health Organization (2017) Depression and other common mental disorders: Global Health Estimates. Geneva
Wunram HL, Hamacher S, Hellmich M, Volk M, Jänicke F, Reinhard F et al (2018) Whole body vibration added to treatment as usual is effective in adolescents with depression: a partly randomized, three-armed clinical trial in inpatients. Eur Child Adolesc Psychiatry 27:645–662. https://doi.org/10.1007/s00787-017-1071-2
Yüksel C, Öngür D (2010) Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. https://doi.org/10.1016/j.biopsych.2010.06.016
Zalsman G, Oquendo MA, Greenhill L, Goldberg PH, Kamali M, Martin A et al (2006) Neurobiology of depression in children and adolescents. Child Adolesc Psychiatr Clin N Am 15:843–868. https://doi.org/10.1016/j.chc.2006.05.010
Acknowledgements
We thank Elena Borovik, Elena Steinbach, Birte Schulte and Marie Hohmann, for their assistance in neurophysiological data collection. Julian Koenig is thanked for proofreading the article. The intervention study could not have been realized without the work of the entire “Balancing Vibrations” team (in alphabetical order): Batool Alkhalil, Miriam Breuer, Lukas Fellner, Jonas Günther, Johanna Jeromin, Svea Loskant, Ruben Matz, Niklas Ploenes, Kathrin Pibiri, Juliana Sciabica, Viktoria Tänzer, Sebastian Wozny, Aaron Yared.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Marga und Walter Boll Foundation (Grant no.: 210-04.00-16) to SB, HLW and NG. The Marga und Walter Boll Foundation was not involved in the design and conduction of the study.
Author information
Authors and Affiliations
Contributions
The study was conceptualized and designed by SB and funding was acquired by HLW, NG and SB. LB conducted the study and was responsible for the study organization. HLW supervised the study conduction. Data were acquired by LB, LP, and EB. LB was responsible for data analysis and interpretation, which was supervised by TAJ and SB. LB performed the statistical analysis, wrote the manuscript, and visualized the data. SB and LB critically revised the manuscript. All authors edited the manuscript and gave final approval for the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Ethical approval
The study was approved by the local ethics committee of the Faculty of Medicine, University of Cologne, Germany and was performed in accordance with the Declaration of Helsinki. All minors and their legal guardians provided written informed consent.
Submission declaration
The manuscript has not been published previously and is not under consideration for publication elsewhere. All authors gave final approval of the version to be published and the manuscript, if accepted, will not be published elsewhere without the written consent of the copyright-holder.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biermann, L., Wunram, H.L., Pokorny, L. et al. Changes in the TMS-evoked potential N100 in the dorsolateral prefrontal cortex as a function of depression severity in adolescents. J Neural Transm 129, 1339–1352 (2022). https://doi.org/10.1007/s00702-022-02539-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02539-9