Abstract
There are contradictory publications and reports regarding the dependence liability of the 3-hydroxy-benzo-1,4-diazepine derivative lormetazepam, one of the most often prescribed hypnotic benzodiazepines which is now also available as an intravenous (i.v.) product for anesthetists. The author was involved in the preclinical and subsequently in the clinical development and post-marketing surveillance of lormetazepam. Here, he reviews the published and unpublished data about lormetazepam dependence and proposes explanations for contradictory views from other authors. On this basis and in contrast to class labeling from regulatory bodies and WHO, the author comes to the conclusion that use of lormetazepam definitely carries a lower risk of inducing dependence and causing abuse than most other benzodiazepines. This applies as well to Sedalam®, the new i.v. application form of lormetazepam, which is much better tolerated than propofol. Because of its pharmacokinetic properties and because all its effects can be fully antagonized with the benzodiazepine antagonist flumazenil, this innovative intravenous application form of lormetazepam provides an excellent method for premedication, symptomatic treatment of excitation and anxiety in the context of surgical or diagnostic procedures including outpatient interventions and for basic sedation during anesthesia.
Similar content being viewed by others
Introduction
Lormetazepam (code ATC: N05CD06) is a very low-dosed 3-hydroxy-benzo-1,4 diazepine benzodiazepine with a terminal half-life of 8–12 h and no active metabolites. After its first approval in Germany in 1980, lormetazepam has been marketed in its oral form (1 mg and 2 mg) as a hypnotic by former Schering AG/Berlin Germany (later by Bayer AG/Leverkusen Germany) as Noctamid® and subsequently in most European countries (trade names in other European countries also include Loramet®, Loretam®, Ergocalm® and others). Lormetazepam still is amongst the three most frequently prescribed benzodiazepine hypnotics in a number of countries. As there have been no marketing activities for lormetazepam for many years, its frequent prescription must be linked to its inherent properties: it has an intermediate terminal half-life of 10 ± 2 h and no active metabolites (Hümpel et al. 1979); so, it does not cause hang-over symptoms on the next morning or day, as they occur with long-acting benzodiazepine hypnotics such as flurazepam. Lormetazepam also does not cause an early rebound as does, e.g., triazolam, but can be considered as a very well-balanced hypnotic drug.
This review intends to demonstrate that lormetazepam differs very favorably from other benzodiazepines also in regard to the risks of dependence and abuse. This unique profile means the new i.v. application in particular is an excellent product to induce anxiolytic and sedative effects wherever this is needed, such as for patients in intensive care units (ICUs).
Pharmacology
In its pharmacology, lormetazepam has been shown to bind in a highly specific way and with high affinity at central benzodiazepine receptors (Dorow et al. 1982) which are located on inhibitory GABAA receptors controlling chloride ion channels. In a radio-receptor assay (where instead of just measuring the serum concentration of given drug, one measures the combined efficacy of a drug and its active metabolites), lormetazepam did bind with similar efficacy as lorazepam and flunitrazepam and with much higher affinity than nitrazepam, flurazepam and diazepam, which correlates very well with the clinical effective hypnotic dosage (Dorow 1987). At the benzodiazepine receptors, similar to other benzodiazepine receptor agonists, lormetazepam enhances the inhibitory GABAergic effects to the physiological maximum. As a result of this indirect mechanism, lormetazepam cannot in fact be overdosed. This also applies to the risk of abuse which is less than with opiates, although both classes of drug eventually enhance the euphoriant and rewarding effects of dopamine.
Toxicology
Due to its indirect mechanism of action, lormetazepam is extremely well tolerated with a very high LD50 of 1.4–2.0 g/kg after oral and intraperitoneal application in mice and even higher than 5 g/kg in rats. In dogs and monkeys, with oral doses as high as 2 g/kg, no lethal effects have been observed. In vitro and in vivo studies with lormetazepam gave no evidence for any mutagenicity, and there were also no embryotoxic or teratogenic effects. Conventional long-term toxicity studies in rats, as well as the carcinogenicity studies, did not show any organ toxicity. In carcinogenicity studies in mice and in rats with lormetazepam dosages of 0.5 mg/kg, 5.0 mg/kg and up to 50 mg/kg, there was no evidence for any tumorigenic effects or, indeed, irreversible organ damage (all data from the German Information for Specialists, under control by the German institute for medicinal drugs and medical products BfArM). All these data have to be seen in the context of the pharmacological effects of lormetazepam in rodents which already could be observed at oral and parenteral dosages as low as 0.05 mg/kg. This makes lormetazepam one of the most potent benzodiazepines known. In the mouse test of reduction of motor activity (which indicates sedative–hypnotic properties), lormetazepam was five times more effective than lorazepam and ten times more effective than the previous standard drugs flurazepam and diazepam. Lormetazepam also shows good anti-convulsant effects in all animal tests. The pharmacological effects of lormetazepam are similar to those of other benzodiazepines (or more precisely, benzodiazepine receptor agonists as there is also a benzodiazepine antagonist, flumazenil, which specifically antagonizes all benzodiazepine agonist effects and which also has a benzodiazepine structure).
The pharmacological effects of lormetazepam are:
-
(1)
Anxiolytic effects (with the indication anxiety and, in case of overdose, with the adverse effects euphoria and dependence liability), and at slightly higher dosages:
-
(2)
Sedative–hypnotic effects (with the clinical indication insomnia and the adverse effects of drowsiness and severe somnolence), and again at slightly higher dosages:
-
(3)
Muscle-relaxant effects (with the clinical indications spasticity and tetanus and, at higher dosages the adverse event ataxia), and at slightly higher doses
-
(4)
Anti-convulsant effects (with the clinical indication grand mal seizures) and
-
(5)
Anterograde amnesia (which at lower dosages occurs only rarely in patients but is more frequent at higher dosage).
However, it has to be kept in mind that all these effects are not absolute (as is the case with, e.g., barbiturates) but the pharmacological effects of a benzodiazepine product are also influenced by the type of patient treated and by their situation.
In the context of this review, only one preclinical effect of lormetazepam needs to be further evaluated, i.e., its dependence liability. This important topic is the aim of studies of benzodiazepines in rhesus monkeys by Tomoshi Yanagita, the leading international expert on dependence in these days, and his group at the Central Institute for Experimental Animals in Kawasaki/Japan. In barbital-dependent rhesus monkeys, even at the very high dose of 256 mg, lormetazepam caused only an incomplete suppression of barbital withdrawal symptoms; whilst, a full suppression of withdrawal symptoms was observed in the same test with 10–12 mg of lorazepam and with 2 mg of nitrazepam (Yanagita et al. 1985). As studies with 3H- (tritiated)-lormetazepam have shown that lormetazepam in monkeys is absorbed at close to 100% (Girkin et al. 1980), a poor bioavailability of this drug in monkeys cannot be the reason for this marked difference. It should be obvious that these data from monkeys give strong support to our argument that lormetazepam has a lower dependence liability than many other benzodiazepines investigated in this validated primate model.
Pharmacokinetics
Lormetazepam does not interfere with the metabolism of other drugs as it only undergoes a phase-II reaction; so, it is just eliminated by 3-OH-glucuronidation and subsequent renal excretion as lormetazepam-3-O-glucuronide and only about 6% of lorazepam-3-O-glucuronide which is inactive (Hümpel et al. 1980). It can, however, show pharmacologic synergies with other sedative–hypnotic compounds including alcohol.
The pharmacokinetic data in the case of lormetazepam could be further validated by studying the bioavailability of lormetazepam in the brain of human volunteers, because benzodiazepines elicit a very typical EEG pattern, i.e., an increase in the beta1-frequency which closely parallels the lormetazepam plasma concentrations and, therefore, proves brain availability of the drug (Kurowski et al. 1982). Obviously, this could also be useful for monitoring lormetazepam efficacy during or after its intravenous infusion or injection in the context of anesthetic procedures.
For this reason, lormetazepam will also produce no or very little hang-over effects which could interfere with, e.g., driving a car or doing work that requires full alertness and concentration. The same holds true for the sublingual administration of lormetazepam, which results in similar pharmacokinetics as tablets (Luscombe 1984); therefore, crushed tablets could be used as premedication for interventions in the stomach or small intestine when these need to be empty. Lormetazepam is also much less likely than triazolam (or alcohol, which also often is used as an hypnotic) to cause rebound phenomena already during the night such as sudden episodes of full alertness, excitation, anxiety, amnesia or even a propensity towards homicide or suicide (so-called Van der Kroef syndrome). In contrast to most other hypnotics including flurazepam and triazolam, the absence of active metabolites in the case of lormetazepam makes dosing on repeated administration much easier, and there is no metabolic interaction with other drugs.
Renal insufficiency can prolong the plasma half-life of lormetazepam-3-hydroxy-glucuronide, but even severe uremia does not alter the distribution and terminal half-life of lormetazepam; whereas, cmax and AUC are reduced, with an accumulation of lormetazepam-3-O-glucuronide by a factor of 5–6; neither is there is any change in the clinical effects of this drug in patients undergoing dialysis (Kampf et al. 1981).
As long as the liver can glucuronidate 250–400-mg bilirubin per day, as shown by the absence of jaundice, an additional 1–2 mg of lormetazepam can be glucuronidated quite easily as well, without causing a burden to the liver either in healthy people or even in patients with liver cirrhosis, as shown by Hildebrandt et al. (1990).
A drug’s pharmacokinetics plays also a great role, with short-acting drugs such as triazolam causing immediate rebound symptoms already during the night and diazepam including its active metabolite (half-lives ranging from 20 to 100 h) accumulating with repeated administration; indeed, with long-term use of this drug and tolerance development, several month after cessation the intake of diazepam, a sudden and single epileptic fit can be the only withdrawal symptom (and cause clinicians to undertake a futile search for a brain tumor or epilepsy). Lormetazepam is in between both extremes; there is no development of tolerance after prolonged administration and only 1 or two days of rebound insomnia, which is why it is recommended to taper this drug off over two days before full ending the treatment (Oswald et al. 1982).
Risk of dependence in humans
General situation
Regarding dependence liability, what is the situation of lormetazepam in humans? First one must know the definition of dependence in humans. It is based on an increased interest in obtaining a given drug, which becomes central to a person’s thoughts, emotions and activities, with the neglect of previous interests, and upon withdrawal causing psychic symptoms such as restlessness, nervousness, irritability and anxiety (= psychological dependence), or additional physical symptoms such as sweating, tremor, tachycardia and in a worst case even convulsions (= physical dependence). If in the case of an anxiolytic there is increased anxiety upon withdrawal, or in the case of a hypnotic there are sleep disturbances as leading withdrawal symptoms, patients will frequently consider these so-called rebound symptoms a re-occurrence of their original complaints and will thus start their treatments again, often even at higher dosages than before. These so-called rebound phenomena—which can exceed the earlier condition in severity—may lead to the development of a severe psychological dependence. Nowadays, one prefers the term substance use disorder (SUD) for both forms of dependence, for which there is a comprehensive checklist in the Diagnostic Manual V of the American Psychiatric Association (2013). Sometimes, the development of tolerance is mistaken for dependence, but these are two completely different qualities of a drug. On the other hand, dependence will not automatically include a trend towards increasing dosages; with benzodiazepines, one usually encounters low-dose dependence. As a rule, benzodiazepines also very rarely cause criminal acts being committed to obtain the drug, since benzodiazepine-dependent people rather tend to rely on compliant doctors, doctor shopping, having others to share prescriptions, etc. In contrast to opiates such as heroin, there is also essentially no physical harm or organ damage.
The frequency of cases of dependence of a given drug is not a fixed number but depends very much on a patient’s personality and the patient population investigated. Doctors and other health care workers are quite susceptible to develop drug dependence and abuse, probably as a result of the easy availability of these drugs for them, but also as a result of the great stress associated with their work. Equally relevant factors are not only the patients’ actual and previous situation, the indication, the dosage and the duration of treatment but also the intrinsic activity and the galenic formulation of a drug, as well as the ways and kinetics of its application and the setting of the use (all these factors are well known to be important also with addiction to opiate drugs). Last but not least, a major factor for inducing dependence is the use of a benzodiazepine in combination with other dependence-inducing drugs, especially with alcohol.
Basically, all benzodiazepines are equal, especially in the eyes of non-clinicians who like to treat all drugs with related chemical and pharmacological properties as identical. Thus, all benzodiazepine hypnotics and anxiolytics have the same warnings regarding potential dependence and abuse approval. Basically, all benzodiazepine compounds have an identical warning information in their package insert and all of them also fall into category IV of the Controlled Substances register by WHO. But even at the WHO, some benzodiazepines are more equal than others: the WHO committee felt compelled to “upgrade” flunitrazepam into category V of their Controlled Substances register because this special benzodiazepine had been used quite often not only as a “date rape drug” but also has a higher general abuse liability as shown in comparative double-blind studies in drug addicts (Mintzer and Griffiths 1998, 2005), possibly due at least in part to active metabolites of flunitrazepam with a long half-life (Dinis-Oliveira 2017). It is also the benzodiazepine found most often in forensic medicine in suicide victims, in cases of murder or in criminals who had been induced by their bosses with flunitrazepam to become uncontrolled killers with no concern for others, neither anxiety nor, indeed, inhibitions (Jones et al. 2016). It seems that flunitrazepam has a very high intrinsic activity, whilst other benzodiazepines are less active in this respect, up to the beta-carboline derivative abecarnil from Schering AG which was shown to be a partial agonist by Mumford et al. (1995). This contrasts with alprazolam, an agonist with full intrinsic activity (similar to flunitrazepam) which has a high dependence liability and which in the US is the benzodiazepine most often involved or co-involved in drug overdose deaths (Heedegard et al. 2018). As flunitrazepam has never been approved in the US, alprazolam apparently has taken the place of flunitrazepam and also seems to play an exceptional role in this country (Wolf and Griffith 1991). Most recently, alprazolam also has been shown to bind deeper to the benzodiazepine pocket of the GABAA receptor complex than the classical benzodiazepine diazepam (Masiulis et al. 2019). Much earlier, it was shown that flumazenil, a drug with a benzodiazepine structure and high affinity for benzodiazepine receptors, was in fact a potent benzodiazepine receptor antagonist with an albeit short-lasting antagonist activity but no agonist effects at its usual dosage (Whitman and Amrein 1995). Some other drugs with high benzodiazepine receptor affinity such as abecarnil act as a partial agonist (Mumford et al. 1965) and FG 7241 even acts as inverse agonist with strong anxiogenic effects just opposite to those of benzodiazepine agonists (Horowski 2020).
Using a radioreceptor assay, Dorow et al. (1982) and Horowski and Dorow (1982) report half-lives of flunitrazepam (including active metabolites) ranging from 15 to 52 h; this high variability results from a number of metabolic steps probably due to the different P450 systems involved in flunitrazepam metabolism. This makes especially the i.v. use of flunitrazepam quite complicated, as it means that, depending on a patient’s CYP 450 status, there could be a need for a very prolonged surveillance period until the patient is fully awake again and can leave the intensive care unit or the physician’s office. Flunitrazepam also causes a stronger respiratory suppression than lormetazepam and is by far the benzodiazepine most commonly found in intoxications and even in single-drug-induced mortality including suicide. These differences suggest that benzodiazepines can have different intrinsic activities and indeed, higher dosages of the benzodiazepine antagonist flumazenil are needed to antagonize the effects of flunitrazepam than in the case of lormetazepam (Suttmann et al. 1990). Further confirmation comes from another leading expert: the results from Yanagita’s group have been repeated, extended and validated by Roland Griffiths from the Johns Hopkins Hospital in Baltimore MA. In addition to his own studies in rhesus monkeys, he and his coworkers did studies with benzodiazepines and other drugs also in healthy volunteers and in drug addicts. In testing the latter, he found out how much money these drug-experienced persons would pay for a given product. He could establish very clearly in this way that flunitrazepam had the highest so-called street value, followed by triazolam and alprazolam and then by diazepam. In agreement with epidemiological data, lorazepam also had a high value; whilst with oxazepam in some of his tests, there was no significant difference from placebo. As lormetazepam so far has not been approved in the US, this compound was not tested by the Johns-Hopkins group; one can assume, however, that it would fall into the oxazepam category (and, indeed, Roland Griffiths, a leading expert for drug abuse, has confirmed this when the author, long time ago, met him at his hospital). These results confirming the animal data are evidence that—in contrast to the position taken by the regulatory bodies—there are significant and relevant differences in the dependence liability of different benzodiazepines not just in animals, but also in humans.
The problem with Minias®
However, there appears to be a problem: if one enters lormetazepam and dependence into the PubMed research system (run by the US government resp. the US National library of Medicine of the NIH which gives scientific and medical abstracts and full texts), one finds four hits within the publications of the twentieth century, all by Italian authors, and all of which describe a very high dependence potential of lormetazepam, much higher than with any other benzodiazepine drug, whether used as an anxiolytic or as a hypnotic. Professor Fabio Lugoboni, one of these authors and head of the specialized Chief Addiction Unit at Verona, can even be heard on YouTube where he warns strongly against the very popular Italian lormetazepam product Minias®. Minias®, which used to be a product of the Schering affiliation Farmades at Rome, nowadays, is a product of a number of Italian generics companies, the owner of one of whom is also active worldwide. In his addiction data base, Lugoboni has found that the by far greatest number of benzodiazepine addicts use Minias® with the active pharmaceutical ingredient lormetazepam. At first glance this is extremely shocking—but why only in Italy? If one looks at Minias®, one will be surprised to see that it is not tablets but drops. Now, it so happens that the former medical director of Farmades, Professor Giovanni Ceccarelli, told the author many years ago that this results in a somewhat faster absorption from the stomach and from the intestines, as indeed published by him and his coworkers (Zecca et al. 1986; confirmed by Ancolio et al. 2004). Ceccarelli added that lormetazepam drops also can be dosed more exactly (including what he called “magic thinking”, i.e., that seven drops bring luck whilst eight is a bad number). One can then find out that the galenical formulation of Minias® is very unusual: it contains sodium saccharine (i.e., Minias® has a very sweet taste), orange aroma (for an aromatic taste), concentrated lemon juice, caramel aroma (as in candy), glycerol, propylene glycol and last but not least a not insignificant amount of alcohol—in other words, quite the cocktail. Unfortunately, a faster absorption of a benzodiazepine might also mean a greater risk of developing dependence, though this is not the only explanation for this Italian epidemic of lormetazepam dependence and addiction. Later, the author learned from other Italian colleagues that due to these qualities of Minias®, Italian heroin addicts add lemon juice, warm this solution up and inject it to themselves to better cope with withdrawal symptoms or to replace opiates for a longer period of time, when these are not available or cannot be afforded. Widespread use or predominantly abuse of Minias® is even supported by its new finding in a waste-water plant in Verona (Repice et al. 2013).
This severe abuse is not singular and can also occur with other benzodiazepines; just as one example, Hayashi et al. (2013) report for Bangkok/Thailand a high rate of intravenous midazolam addiction with all associated harm, where this product also is being used in most cases as a substitute for heroin. In conclusion—and as conceded by a paper from Lugoboni’s Verona group (Faccini et al. 2019) discussing a strange case of “Jekyll and Dr. Hyde”—it is the galenic formula and not the active ingredient of lormetazepam which causes this abuse. For these reasons, these Italian findings are not relevant for the evaluation of the dependence risk associated with lormetazepam.
It is strange, however, that even nowadays, generic Minias® imitations are on the Italian market, including a product from Bayer Italy Spa., the Italian affiliation of Bayer AG in Germany/Leverkusen. Apparently, this application form still sells very well. The people who had developed Minias® could have learned about the inherent dangers of their Minias® galenic application form from a precedent with another benzodiazepine drug, i. e., the case of the temazepam abuse where the benzodiazepine is dissolved in a solution within a soft gelatin capsule; it, thus, was and still is very easy for heroin addicts to inject themselves with this solution (Brin et al. 2004; Dwyer 2008).
Dependence and abuse liability of other benzodiazepines
From the PubMed system which has been mentioned already, one can get even more information by combining the search item “abuse” with the names of different benzodiazepines of interest. It goes without saying that this is not a terribly scientific method but, nevertheless, it yields quite interesting findings:
Our search brings up 2108 hits for diazepam, alprazolam 489, zolpidem 404, flunitrazepam 397, midazolam 370, propofol 321, oxazepam 300, zopiclone 244, temazepam 187 and lorazepam 148. And lormetazepam? One finds a total of just 28 quotations. To be fair, a small percentage of these quotations might refer to the use of a benzodiazepine to mitigate symptoms from another drug of abuse such as heroin, but these will not really change the general picture.
There is an even lower number of hits for lormetazepam, i.e., only 7, if one replaces “abuse” with “dependence”, but the latter word has a number of other medical meanings. Anyway, in this case, diazepam still gets 641 hits, lorazepam 148 and midazolam 109, which is still 13 times higher than lormetazepam. Even among these seven publications, no fewer than 4 are from the Verona group discussed above; while, the others only mention lormetazepam with a number of other hypnotics in the context of general recommendations for use. By this metric, only temazepam comes close to lormetazepam with 29 hits (which still is three times higher than lormetazepam). Most experts would agree, however, that temazepam, because it is slow to enter the brain, is a poor hypnotic—which gives lormetazepam a much better benefit/risk ratio.
One could object that these data must be corrected with the respective sales of these drugs but with lormetazepam over many years having been one of the leaders amongst the benzodiazepine hypnotics, it will make this drug looking even more favorable. Here, the author will give just one example from the German public health insurances in 2016 (Schwabe and Paffrath 2016): there were 263,000 prescriptions for lormetazepam, 292,000 for alprazolam and 193,000 for temazepam.
The benzodiazepine agonists zolpidem and zopiclone have higher sales but they will be not further discussed here as they are available only in oral form; furthermore, these drugs can induce, although very rarely, very severe psychiatric adverse events and sometimes even death by a voluntary or involuntary overdose, which in the US has caused the FDA to implement a black box warning for the two drugs, zolpidem and eszopiclone (the S-isomer of zopiclone instead of the racemate). There are also metabolic interactions via the CYP 3A4 system, in contrast to the 3-hydroxy-benzodiazepines (Greenblatt et al. 1998; Greenblatt and Zammit 2012). The claim that they have a lower risk for inducing dependence has not been substantiated (Gunja 2013; Hoffmann and Glaeske 2014; Schiffano et al. 2019). All these data support our view that not all benzodiazepines are equal and that lormetazepam has one of the lowest risks of dependence amongst this class of drugs.
An isolated divergent opinion
In Germany, Wolfgang Poser, MD, psychiatrist and assistant professor from Göttingen University, has taken a very strong position against any use of benzodiazepines in whatever indication (Kemper et al. 1980; Poser and Poser 1996). In the publication of 1996, one can see that Poser in 1991 has analyzed a consecutive series of 2127 patients from the Göttingen university hospital over 17 years who had some form of drug dependence. Amongst them, he has found 1196 cases of benzodiazepine dependence, in most cases, however, combined with alcohol abuse. 141 patients had a “pure” benzodiazepine dependence, around 90 of them on diazepam and 40 on lorazepam. Lormetazepam, despite being the market leader amongst all hypnotics in this time, was only mentioned as one of the “others”, i.e. among those drugs with only between 1 and 6 cases detected within these 17 years. This, if anything, just confirms our position that lormetazepam monotherapy has a very low risk of dependence.
It must be emphasized that Professor Poser has used a very narrow definition, i.e., just patients continuing such a treatment beyond 4 weeks.
Reports to former Schering AG
Subsequently, the majority of cases reported to Schering AG, then the only provider of lormetazepam, in the years 1979–1990, which have been received by the author (amongst a total of only 30 cases), came from Professor Poser’s hospital; I vividly recall the case of one female doctor with terminal cancer who used 2-mg lormetazepam every evening to cope with her situation and who, very understandably in my view, flatly refused to stop intake after 4-week treatment, and instead continued the intake of low-dosed lormetazepam until her death a few weeks later.
I have no doubt that Wolfgang Poser would take her as another case of evidence of lormetazepam dependence. Most of the other cases I remember were reports about combinations of lormetazepam with alcohol; indeed, it is well known that an alcoholic would need less of the expensive alcohol if he manages to convince a doctor to prescribe him a benzodiazepine against his—of course alcohol-induced—insomnia or anxieties.
To conclude this: as the author was the person with the medical responsibility, all these cases immediately came to his desk for a final evaluation once they had reached our department of drug safety. In addition to that, regular monthly conferences for the evaluation of all new or major adverse events, guaranteed that not a single report could have been overlooked. Thus, as the clinician who was in charge of lormetazepam for some 30 years, the author can confirm that compared to other benzodiazepines, the dependence potential of oral lormetazepam is extremely low.
Lormetazepam i.v.
Returning to the importance of a drug’s pharmacokinetics, it is well known that the speed of CNS penetration plays an obvious role in the dependence liability of a given product, as the “kick” drug addicts experience plays a major role. This is demonstrated very clearly by the differences between oral morphine and parenteral morphine (and even more so if one looks at heroin), because the faster an addictive drug gets into the CNS, the greater usually is its abuse potential. Thus, one has to give i.v. lormetazepam a separate evaluation, given that in theory there could be a greater “kick” by such an injection. Needless to say, however, there also was not a single case of drug dependence reported about Schering’s parenteral lormetazepam (Noctamid® i.v.). The answer why this is the case is easy: because of its prominent local side effects which included severe pain at the injection site, drug addicts never liked Noctamid i.v.®. These side effects are due also to its galenic constitution, with 10-ml vials containing 5 ml of propylene glycol as a solvent, which has made this product extremely hyperosmolar. The first volunteer (RH) has experienced this: when injected into a large cubital vein, Noctamid® i.v. caused a severe local pain “like a knife inserted into the vein” with immediate hemolysis, as one could see from the dark red serum in the blood taken from the other arm where blood was collected for pharmacokinetic studies. Subsequently, the urine had the color of black cherries and within the next days, there was a long-lasting venous local inflammation and thrombosis. It goes without saying that even the most desperate drug addict will not want to repeat such a painful experience. Due to these local reactions, the duration of the clinical use of Noctamid i.v.® had been greatly restricted.
Sedalam
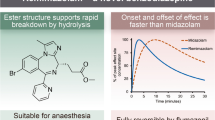
As a result of the severe local adverse effects of Noctamid i.v.® and despite its good general efficacy as an anesthetic, very soon this product was no longer marketed by Schering AG, but given to the late anesthetist Rainer Hoernecke from Munich who had asked for it for further development. It was his firm belief that lormetazepam with its outstanding general safety and especially with the availability of an i.v. form would be a great progress in countries with very poor access to medical doctors. He, thus, hoped to make it available to developing countries—for this reason, he already had established contacts to the department of health of the government of the Philippines. He was as convinced as the author that with a better i.v. application form of lormetazepam, even non-medical people could provide patients with strong anxiolysis, deep sedation and anesthesia and thus relieve them from anxiety and pain as well as even save, e.g., in complicated childbirth, a great number of lives in rural parts of developing countries where there are no or only very few MDs. After many attempts, Rainer Hoernicke came up with an innovative new galenic formulation which is more than just the addition of its parts (Hoernicke 2011). This has become an excellent hypnotic/anesthetic from Dr. Köhler-Chemie/Bensheim, which is called Sedalam® and which can always be combined with additional oral (or even sublingual) premedication with lormetazepam tablets without causing any metabolic interaction. Sedalam® has been used successfully in intensive care over many days, where all other attempts to induce anxiolysis and reversible sedation had failed, and this could be achieved without any drug accumulation, as shown by the Charité’s anesthetists around professor Claudia Spies (Lüth et al. 2014). Their findings have been confirmed in other institutions and in a very large multicenter study comparing the new i.v. formulation with i.v. midazolam (C. Spies, pers. comm.). Most recently, the great safety associated with Sedalam® can be expected to prove very helpful in providing sedation and reducing anxiety when intensive care units have increasingly problems to cope with increasing numbers of patients with pneumonia, e.g., caused by the new corona virus.
In the context of this review, one also has to discuss what risk for dependence this latest i.v. application form of lormetazepam, i.e., Sedalam®, may have. First of all, this product is only administered under the direct control of a doctor or more often in a hospital and especially in ICUs; but even if someone such as a hospital worker (a group among whom, due to great stress and difficult working conditions, there is a high incidence of drug dependence, though usually from “harder” drugs, Warner et al. 2013) were to inject himself with this product, the immediate sedation and, most often, the anterograde amnesiogenic effect of lormetazepam will remove all the abuser’s memories of whatever pleasant feelings (if any) there might have been. Therefore, the dependence liability and risk of abuse of lormetazepam in whatever application form is very low and clearly lower than in the case of most other benzodiazepines. Furthermore, whatever happens can be reverted immediately with flumazenil. Also, under the conditions under which Sedalam® is being used, there is virtually no risk that patients have access to alcohol, which otherwise, when combined with lormetazepam, would present a problem. Based upon the pharmacology and vast clinical experience with oral lormetazepam over 40 years, also no new severe adverse events need to be expected with this new intravenous lormetazepam product. This is in marked contrast to the situation of propofol which is being used by anesthetists in similar indications; with this drug, a very rare but severe adverse event, i.e., the propofol infusion syndrome, quite recently has been reported which consists of fever, rhabdomyolysis, metabolic acidosis, hyperkalemia, ECG changes and cardiac failure and other symptoms and for which a mortality has been reported ranging from 18% up to about 50% (Hemphill et al. 2019).
Conclusion
In conclusion, not all benzodiazepines are equal: oral lormetazepam, due to its great safety and very low dependence liability, is one of the best to be used as a hypnotic and for premedication; whilst, the risk of abuse is even lower with the new and well-tolerated intravenous lormetazepam product Sedalam®. It differs from midazolam and flunitrazepam as it does not have active metabolites, and therefore, no metabolic interactions are possible. In contrast to flunitrazepam, it does not cause respiratory depression except under very special conditions, and even then only very rarely. Patients are not as heavily sedated as in the case of the other benzodiazepines but still have less anxiety than patients on placebo, propofol or dexmedetomidine. It is better tolerated than propofol which (similar to dexmedetomidine) also shows cardiovascular side effects such as an unstable blood pressure and generally is less safe respectively needs more surveillance.
References
Ancolio C, Tardieu S, Soubrouillard AC, Pradel V, Micallef J, Blin O (2004) A randomized clinical trial comparing doses and efficacy of lormetazepam tablets or oral solution for insomnia in a general practice setting. Hum Psychopharmacol 19:129–134
Breen CL, Degenhardt LJ, Bruno RB, Roxburgh AD, Jenkinson R (2004) The effects of restricting publicly subsidized temazepam capsules on benzodiazepine use among injecting drug users in Australia. Med J Aust 181:300–304
Cosci F, Mansueto G, Faccini M, Casari R, Lugoboni F (2016) Socio-demographic and clinical characteristics of benzodiazepine long-term users: results from a tertiary care center. Compr Psychiatry 69:211–215
Dinis-Oliveira RJ (2017) Metabolic profile of flunitrazepam: clinical and forensic toxicological aspects. Drug Metab Lett 11:14–20
Dorow R, Seidler J, Schneider HH (1982) A radioreceptor assay to study the affinity of benzodiazepines and their receptor binding activity in human plasma including their active metabolite. Br J Pharmacol 13:561–565
Dorow R (1987) Pharmacokinetic and clinical studies with a benzodiazepine radioreceptor assay. Psychopharmacology Suppl 1:105–118 (review)
Dwyer R (2008) Privileging pleasure: temazepam injection in a heroin marketplace. Int J Drug Policy 19:367–374
Faccini M, Tamburin S, Casari R, Morbioli L, Lugoboni F (2019) High dose lormetazepam dependence: strange case of Dr. Jekyll and Mr. Hyde. Intern Emerg Med 14:1271–1278
Girkin R, Baldock GA, Chasseaud LF, Hümpel M, Hawkins DR, Mayo BC (1980) The absorption, distribution and excretion of [14C] lormetazepam in dogs, rabbits, rats and rhesus monkeys. Xenobiotica 10:401–411
Greenblatt DJ, von Moltke LL, Harmatz JS, Mertzanis P, Graf JA, Durol AL, Counihan M, Roth-Schechter B, Shader RI (1998) Kinetic and dynamic interaction study of zolpidem with ketoconazole, itraconazole, and fluconazole. Clin Pharmacol Ther 64:661–671
Greenblatt DJ, Zammit GK (2012) Pharmacokinetic evaluation of eszopiclone: clinical and therapeutic implications. Expert Opin Drug Metab Toxicol 8:1609–1618
Gunja N (2013) The clinical and forensic toxicology of Z-drugs. J Med Toxicol 9:155–162
Hayashi K, Suwannawong P, Ti L, Kaplan K, Wood E, Kerr T (2013) High rate of midazolam injection and associated harms in Bangkok, Thailand. Addiction 108:944–952
Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M (2018) Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep 67:1–14
Hemphill S, McMenamin L, Bellamy MC, Hopkins PM (2019) Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth 122:448–459
Hildebrandt M, Hellstern A, Hümpel M, Hellenbrecht D, Saller R (1990) Plasma levels and urinary excretion of lormetazepam in patients with liver cirrhosis and in healthy volunteers. Eur J Drug Metab Pharmacokinet 15:19–26
Hoffmann F, Glaeske G (2014) Benzodiazepine hypnotics, zolpidem and zopiclone on private prescriptions: use between 1993 and 2012 (in German). Nervenarzt 85:1402–1409
Hoernecke R (2011) US patent US20110263576A1
Horowski R, Dorow R (1982) Significance of pharmacokinetic findings for the clinical effect of benzodiazepines. Internist (Berl) 23:632–640
Horowski R (2020) FG7142: is this validated tool to study anxiety now forgotten? J Neural Transm 127:287–289
Hümpel M, Illi V, Milius W, Wendt H, Kurowski M (1979) The pharmacokinetics and biotransformation of the new benzodiazepine lormetazepam in humans. I. Absorption, distribution, elimination and metabolism of lormetazepam-5-14C. Eur J Drug Metab Pharmacokinet 4:237–243
Hümpel M, Nieuweboer B, Milius W, Hanke H, Wendt H (1980) Kinetics and biotransformation of lormetazepam. II. Radioimmunologic determinations in plasma and urine of young and elderly subjects: first-pass effect. Clin Pharmacol Ther 28:673–679
Hümpel M, Stoppelli I, Milia S, Rainer E (1982) Pharmacokinetics and biotransformation of the new benzodiazepine, lormetazepam, in man. Eur J Clin Pharmacol 21:421–425
Jones AW, Holmgren A, Ahlner J (2016) Post-mortem concentrations of drugs determined in femoral blood in single-drug fatalities compared with multi-drug poisoning deaths. Forensic Sci Int 267:96–103
Kampf D, Hümpel M, Lurche U, Kessel M (1981) The effects of uremia and hemodialysis on lormetazepam disposition. Clin Pharmacol Ther 30:77–85
Kemper N, Poser W, Poser S (1980) Benzodiazepine dependence: addiction potential of the benzodiazepines is greater than previously assumed. Dtsch Med Wochenschr 105:1707–1712
Klotz U, Duka T, Dorow R, Doenicke A (1985) Flunitrazepam and lormetazepam do not affect the pharmacokinetics of the benzodiazepine antagonist Ro 15–1788. Br J Clin Pharmacol 19:95–98
Kurowski M, Ott H, Herrmann WM (1982) Relationship between EEG dynamics and pharmacokinetics of the benzodiazepine lormetazepam. Pharmacopsychiatria 15:77–83
Luetz A, Weiss B, Spies CD (2014) Intravenous lormetazepam during sedation weaning in a 26-year-old critically ill woman. Case Rep Crit Care 2014 art. ID 372740
Lugoboni F, Mirijello A, Morbioli L, Faccini M, Casari R, De Cosmo S, Gasbarrini A, Addolorato G (2019) Zolpidem high-dose abuse: what about the liver? Results from a series of 107 patients. Expert Opin Drug Saf 18:753–758
Luscombe DK (1984) Lormetazepam–plasma concentrations in volunteers following sublingual and oral dosing. Psychopharmacology 1:99–104
Maier C, Iwunna J, Tsokos M, Mußhoff F (2017) Deaths from propofol abuse: survey of institutes of forensic medicine in Germany, Austria and Switzerland. Anaesthesist 66:109–114
Masiulis S, Desai R, Uchański T, Serna Martin I, Laverty D, Karia D, Malinauskas T, Zivanov J, Pardon E, Kotecha A, Steyaert J, Miller KW, Aricescu AR (2019) GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565:454–459
Mintzer MZ, Griffiths RR (1998) Flunitrazepam and triazolam: a comparison of behavioral effects and abuse liability. Drug Alcohol Depend 53:49–66
Mintzer MZ, Griffiths RR (2005) An abuse liability comparison of flunitrazepam and triazolam in sedative drug abusers. Behav Pharmacol 16:579–584
Mumford GK, Rush CR, Griffiths RR (1995) Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther 272:570–580
Oswald I, French C, Adam K, Gilham J (1982) Benzodiazepine hypnotics remain effective for 24 weeks. Br Med J (Clin Res Ed) 284:860–863
Poser W, Poser S (1996) Medikamente—Missbrauch und Abhängigkeit (in German). Thieme Stuttgart/New York
Raffa RB, Cavallo F, Capasso A (2007) Flumazenil-sensitive dose-related physical dependence in planarians produced by two benzodiazepine and one non-benzodiazepine benzodiazepine-receptor agonists. Eur J Pharmacol 564:88–93
Repice C, Dal Grande M, Maggi R, Pedrazzani R (2013) Licit and illicit drugs in a wastewater treatment plant in Verona. Italy Sci Total Environ 463(464):27–34
Schiffano F, Chiappini S, Corkery JM, Guirguis A (2019) An insight into Z-drug abuse and dependence: an examination of reports to the European Medicines Agency database of suspected adverse drug reactions. Int J Neuropsychopharmacol 22:270–277
Schwabe U, Paffrath D (eds) (2016) Arzneiverordnungsreport (in German). Springer, Berlin
Suttmann H, Rampf U, Juhl G, Greim M, Doenicke A (1990) Beta-Aktivierung nach intravenöser Gabe von Benzodiazepinen und dem spezifischen Antagonisten Flumazenil (in German). Z EEG-EMG 21:20–28
Tetzlaff J, Collins GB, Brown DL, Leak BC, Pollock G, Popa D (2010) A strategy to prevent substance abuse in an academic anesthesiology department. J Clin Anesth 22:143–150
Weerts EM, Kaminski BJ, Griffiths RR (1998) Stable low-rate midazolam self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Psychopharmacology 135:70–81
Warner DO, Berge K, Sun H, Harman A, Hanson A, Schroeder DR (2013) Substance use disorder among anesthesiology residents, 1975–2009. JAMA 310:2289–2296
Whitwam JG, Amrein R (1995) Amrein R (1995) Pharmacology of flumazenil. Acta Anaesthesiol Scand Suppl 108:3–14
Wolf B, Griffiths RR (1991) Physical dependence on benzodiazepines: differences within the class. Drug Alcohol Depend 29:153–156
Woods JH, Katz JL, Winger G (1987) Abuse liability of benzodiazepines. Pharmacol Rev 39:251–413
Yanagita T, Kato S, Mikami M (1985) Dependence potential of lormetazepam studied in rhesus monkeys. Preclin Rep Cent Inst Exp 11:251–413
Zecca L, Reina L, Scaglione F, Ferrario P, Pirola R, Ceccarelli G, Ciampini M, Fraschini F (1985) Relative bioavailability in humans for oral tablets and solutions of lormetazepam. Arzneimittelforschung 35:1870–1872
Acknowledgements
The author thank Peter Riederer for his advice as well as Christian Riederer for his technical and other support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author had been paid by former Schering AG where as an employee until 1999, he also was in charge of lormetazepam. He fully owns Antaxios GmbH, a start-up company specialized in medical expert opinions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horowski, R. Dependence liability of lormetazepam: are all benzodiazepines equal? The case of the new i.v. lormetazepam for anesthetic procedures. J Neural Transm 127, 1107–1115 (2020). https://doi.org/10.1007/s00702-020-02209-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02209-8