Abstract
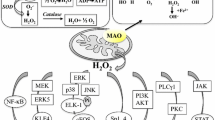
Post-translational influences could underlie the ambiguous roles of monoamine oxidase-A (MAO-A) in pathologies such as depression, cancer and Alzheimer disease. In support of this, we recently demonstrated that the Ca2+-sensitive component of MAO-A catalytic activity is inhibited by a pro-survival p38 (MAPK)-dependent mechanism. We substituted three aspartic acid (D) residues in human MAO-A that reside in putative Ca2+-binding motifs and overexpressed the individual proteins in the human HEK293 cell line. We assayed the overexpressed proteins for catalytic activity and for their influence on cell viability (using MTT conversion and trypan blue exclusion) and proliferation/DNA synthesis [using bromodeoxyuridine (BrdU) incorporation]. Innate MAO-A catalytic activity (and the capacity for generating hydrogen peroxide) was unaffected by the D61A substitution, but inhibited moderately or completely by the D248A and D328G substitutions, respectively. The Ca2+-sensitive activities of wild-type and D248A MAO-A proteins were enhanced by treatment with the selective p38(MAPK) inhibitor, SB203580, but was completely abrogated by the D61A substitution. Monoamine oxidase-A(D61A) was toxic to cells and exerted no effect on cell proliferation, while MAO-A(D248A) was generally comparable to wild-type MAO-A. As expected, the catalytic-dead MAO-A(D328G) was not cytotoxic, but unexpectedly enhanced both MTT conversion and BrdU staining. Variant-dependent changes in Bax and Bcl-2/Bcl-XL protein expression were observed. A different pattern of effects in N2-a cells suggests cell line-dependent roles for MAO-A. A catalytic-dependent mechanism influences MAO-A-mediated cytotoxicity, whereas a catalytic-independent mechanism contributes to proliferation. Context-dependent inputs by either mechanism could underlie the ambiguous pathological contributions of MAO-A.





Similar content being viewed by others
References
Ahmed ZM et al (2008) Gene structure and mutant alleles of PCDH15: nonsyndromic deafness DFNB23 and type 1 Usher syndrome. Hum Genet 124:215–223
Astuto LM et al (2002) CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet 71:262–275
Cao X et al (2007) Calcium-sensitive regulation of monoamine oxidase-A contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology. BMC Neurosci 8:73
Cao X et al (2009a) Calcium alters monoamine oxidase-A parameters in human cerebellar and rat glial C6 cell extracts: possible influence by distinct signalling pathways. Life Sci 85:262–268
Cao X et al (2009b) Serine 209 resides within a putative p38(MAPK) consensus motif and regulates monoamine oxidase-A activity. J Neurochem 111:101–110
Chan-Palay V et al (1993) Calbindin D-28k and monoamine oxidase A immunoreactive neurons in the nucleus basalis of Meynert in senile dementia of the Alzheimer type and Parkinson’s disease. Dementia 4:1–15
Chiou SH et al (2006) Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol 148:587–598
De Colibus L et al (2005) Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proc Natl Acad Sci USA 102:12684–12689
De Zutter GS, Davis RJ (2001) Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Proc Natl Acad Sci USA 98:6168–6173
Du L et al (2002) High activity-related allele of MAO-A gene associated with depressed suicide in males. NeuroReport 13:1195–1198
Emilsson L et al (2002) Increased monoamine oxidase messenger RNA expression levels in frontal cortex of Alzheimer’s disease patients. Neurosci Lett 326:56–60
Filomeni G et al (2007) Trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr 2:295–305
Fitzgerald JC et al (2007) Monoamine oxidase-A modulates apoptotic cell death induced by staurosporine in human neuroblastoma cells. J Neurochem 103:2189–2199
Fowler JS et al (2007) Evidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjects. Biol Psychiatry 62:355–358
Geha RM et al (2001) Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem 276:9877–9882
Green AR et al (1977) Evidence for dopamine deamination by both type A and type B monoamine oxidase in rat brain in vivo and for the degree of inhibition of enzyme necessary for increased functional activity of dopamine and 5-hydroxytryptamine. Br J Pharmacol 60:343–349
Grunblatt E et al (2005) Oxidative stress related markers in the “VITA” and the centenarian projects. Neurobiol Aging 26:429–438
Handschuh G et al (2001) Single amino acid substitutions in conserved extracellular domains of E-cadherin differ in their functional consequences. J Mol Biol 314:445–454
Hiro I et al (1996) Characterization of rat monoamine oxidase A with noncovalently-bound FAD expressed in yeast cells. J Biochem 120:759–765
Inaba-Hasegawa K et al (2011) Type A monoamine oxidase is associated with induction of neuroprotective Bcl-2 by rasagiline, an inhibitor of type B monoamine oxidase. J Neural Transm. doi:10.1007/s00702-011-0730-6
Jungwirth N et al (2008) Serotonin used as prognostic marker of urological tumors. World J Urol 26:499–504
Kennedy BP et al (2003) Early and persistent alterations in prefrontal cortex MAO A and B in Alzheimer’s disease. J Neural Transm 110:789–801
Lin FJ et al (2001) Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol 21:7287–7294
Lizcano JM et al (1991) Amine oxidase activities in rat breast cancer induced experimentally with 7,12-dimethylbenz(alpha)anthracene. Biochem Pharmacol 42:263–269
Mandel SA et al (2007) Rasagiline promotes regeneration of substantia nigra dopaminergic neurons in post-MPTP-induced Parkinsonism via activation of tyrosine kinase receptor signaling pathway. Neurochem Res 32:1694–1699
Meyer JH et al (2006) Elevated monoamine oxidase A levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63:1209–1216
Mousseau DD et al (1997) Increased density of catalytic sites and expression of brain monoamine oxidase A in humans with hepatic encephalopathy. J Neurochem 68:1200–1208
Nandigama RK, Edmondson DE (2000) Influence of FAD structure on its binding and activity with the C406A mutant of recombinant human liver monoamine oxidase A. J Biol Chem 275:20527–20532
Naoi M et al (2011) Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Int Rev Neurobiol 100:85–106
Nishimura AL et al (2005) Monoamine oxidase a polymorphism in Brazilian patients: risk factor for late-onset Alzheimer’s disease? J Mol Neurosci 27:213–217
Ou XM et al (2006) Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci USA 103:10923–10928
Pai VP et al (2009) Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res 11:R81
Peehl DM et al (2008) The significance of monoamine oxidase-A expression in high grade prostate cancer. J Urol 180:2206–2211
Riederer P, Laux G (2011) MAO-inhibitors in Parkinson’s disease. Exp Neurobiol 20:1–17
Rodriguez S et al (2011) Resistance of the golden hamster to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-neurotoxicity is not only related with low levels of cerebral monoamine oxidase-B. Exp Toxicol Pathol. http://dx.doi.org/10.1016/j.etp.2011.06.010
Rybaczyk LA et al (2008) An indicator of cancer: downregulation of monoamine oxidase-A in multiple organs and species. BMC Genomics 9:134
Saura J et al (1994a) Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62:15–30
Saura J et al (1994b) Age-related changes on MAO in Bl/C57 mouse tissues: a quantitative radioautographic study. J Neural Transm Suppl 41:89–94
Saura J et al (1994c) Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol Aging 15:399–408
Shaw G et al (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. Faseb J 16:869–871
Sherif F et al (1992) Brain gamma-aminobutyrate aminotransferase (GABA-T) and monoamine oxidase (MAO) in patients with Alzheimer’s disease. J Neural Transm Park Dis Dement Sect 4:227–240
Sparks DL et al (1991) Alterations in brain monoamine oxidase activity in aging, Alzheimer’s disease, and Pick’s disease. Arch Neurol 48:718–721
Sun A et al (2003) P38 MAP kinase is activated at early stages in Alzheimer’s disease brain. Exp Neurol 183:394–405
Takehashi M et al (2002) Association of monoamine oxidase A gene polymorphism with Alzheimer’s disease and Lewy body variant. Neurosci Lett 327:79–82
Vasquez-Vivar J et al (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95:9220–9225
Wang J et al (2009) Mutagenic probes of the role of Ser209 on the cavity shaping loop of human monoamine oxidase A. FEBS J 276:4569–4581
Wei Z et al (2003) Atypical antipsychotics attenuate neurotoxicity of beta-amyloid (25–35) by modulating Bax and Bcl-X(l/s) expression and localization. J Neurosci Res 74:942–947
Wu YH et al (2007) A promoter polymorphism in the monoamine oxidase A gene is associated with the pineal MAOA activity in Alzheimer’s disease patients. Brain Res 1167:13–19
Yanez M et al (2006) Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun 344:688–695
Yu DS et al (2001) The expression of neuropeptides in hyperplastic and malignant prostate tissue and its possible clinical implications. J Urol 166:871–875
Zhao H et al (2009) Anti-oncogenic and pro-differentiation effects of clorgyline, a monoamine oxidase A inhibitor, on high grade prostate cancer cells. BMC Med Genomics 2:55
Zhu X et al (2003) Oxidative stress and neuronal adaptation in Alzheimer disease: the role of SAPK pathways. Antioxid Redox Signal 5:571–576
Acknowledgments
This work was funded by a departmental Aruna and Kripa Thakur Award (to XC), by the Canadian Institutes of Health Research-Saskatchewan Health Research Foundation (SHRF) Operating Grant (to DDM) and by a Canadian Breast Cancer Foundation-Prairies/NWT Region Research Grant (to DDM). TSM is the recipient of a College of Graduate Studies and Research Master’s Scholarship. DDM holds the Saskatchewan Research Chair in Alzheimer’s Disease and Related Dementias funded jointly by the Alzheimer Society of Saskatchewan and SHRF. There are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Z., Satram-Maharaj, T., Chaharyn, B. et al. Aspartic acid substitutions in monoamine oxidase-A reveal both catalytic-dependent and -independent influences on cell viability and proliferation. J Neural Transm 119, 1285–1294 (2012). https://doi.org/10.1007/s00702-012-0779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0779-x