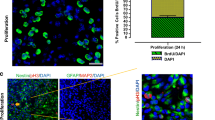
Abstract
The concentrations of soluble β-amyloid (Aβ) oligomers paralleled with the extent of synaptic loss and severity of cognitive impairment in Alzheimer patients. However, the neurotoxicity of the naturally generated Aβ species remains unknown. This study was designed to examine the effects of naturally generated Aβ oligomers, secreted from amyloid precursor protein-expressing cells, on the homeostasis and viability of primary hippocampal neurons. Our results showed that primary hippocampal neurons incubated with condition media containing cell-secreted soluble Aβ had higher levels of heat-shock protein (HSP)27, HSP60 and HSP70, and lower levels of HSP32 than those of the control neurons. The cell-secreted soluble Aβ caused mitochondria dysfunction in hippocampal neurons as demonstrated by depolarized membrane potential and decreased cytochrome c oxidase activity and ATP levels. The levels of pro-apoptotic proteins, Bid, Bax and cytochrome C, were elevated; whereas anti-apoptotic Bcl-2 protein was reduced in the soluble Aβ-cultured neurons. Apoptosis was also evident in these soluble Aβ-cultured neurons. These results indicate that naturally secreted Aβ induces neuronal injury/death by activating an apoptotic pathway involving impaired mitochondria function and cellular homeostasis.




Similar content being viewed by others
References
Anandatheerthavarada HK, Biswas G, Robin MA et al (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161:41–54
Clarimon J, Bertranpetit J, Boada M et al (2003) HSP70-2 (HSPA1B) is associated with noncognitive symptoms in late-onset Alzheimer’s disease. J Geriatr Psychiatry Neurol 16:146–150
Cleary JP, Walsh DM, Hofmeister JJ et al (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8:79–84
Clementi ME, Marini S, Coletta M et al (2005) Abeta(31-35) and Abeta(25-35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: role of the redox state of methionine-35. FEBS Lett 579:2913–2918
Converso DP, Taille C, Carreras MC et al (2006) HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. Faseb J 20:1236–1238
Evans CG, Wisen S, Gestwicki JE (2006) Heat-shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem 281:33182–33191
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hartley DM, Walsh DM, Ye CP et al (1999) Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19:8876–8884
Katzman R, Terry R, DeTeresa R et al (1988) Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23:138–144
Kayed R, Head E, Thompson JL et al (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489
Kuo YM, Emmerling MR, Vigo-Pelfrey C et al (1996) Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 271:4077–4081
Kuo YM, Emmerling MR, Woods AS et al (1997) Isolation, chemical characterization, and quantitation of A beta 3-pyroglutamyl peptide from neuritic plaques and vascular amyloid deposits. Biochem Biophys Res Commun 237:188–191
Kuo YM, Webster S, Emmerling MR et al (1998) Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer’s disease. Biochim Biophys Acta 1406:291–298
Lambert MP, Barlow AK, Chromy BA et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Liao L, Cheng D, Wang J et al (2004) Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 279:37061–37068
Lue LF, Kuo YM, Roher AE et al (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155:853–862
Maes OC, Kravitz S, Mawal Y et al (2006) Characterization of alpha1-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiol Dis 24:89–100
Magrane J, Smith RC, Walsh K et al (2004) Heat-shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci 24:1700–1706
Masliah E, Honer WG, Mallory M et al (1994) Topographical distribution of synaptic-associated proteins in the neuritic plaques of Alzheimer’s disease hippocampus. Acta Neuropathol 87:135–142
McLean CA, Cherny RA, Fraser FW et al (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46:860–866
Mehta PD, Pirttila T, Mehta SP et al (2000) Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol 57:100–105
Morishima-Kawashima M, Ihara Y (1998) The presence of amyloid beta-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 37:15247–15253
Motter R, Vigo-Pelfrey C, Kholodenko D et al (1995) Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol 38:643–648
Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6:11–22
Naslund J, Haroutunian V, Mohs R et al (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283:1571–1577
Nunez J (2008) Primary culture of hippocampal neurons from P0 newborn rats. J Vis Exp. doi:10.3791/3895
Nunomura A, Perry G, Aliev G et al (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60:759–767
Pillot T, Drouet B, Queille S et al (1999) The nonfibrillar amyloid beta-peptide induces apoptotic neuronal cell death: involvement of its C-terminal fusogenic domain. J Neurochem 73:1626–1634
Podlisny MB, Ostaszewski BL, Squazzo SL et al (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem 270:9564–9570
Pratico D, Uryu K, Leight S et al (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 21:4183–4187
Reers M, Smiley ST, Mottola-Hartshorn C et al (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417
Renkawek K, Bosman GJ, de Jong WW (1994) Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol 87:511–519
Renkawek K, Stege GJ, Bosman GJ (1999) Dementia, gliosis and expression of the small heat-shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport 10:2273–2276
Roher AE, Lowenson JD, Clarke S et al (1993) Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J Biol Chem 268:3072–3083
Ryter SW, Alam J, Choi AM (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86:583–650
Schipper HM, Bennett DA, Liberman A et al (2006) Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging 27:252–261
Selkoe DJ (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 192:106–113
Shoji M, Golde TE, Ghiso J et al (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258:126–129
Sponne I, Fifre A, Drouet B et al (2003) Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J Biol Chem 278:3437–3445
Tamaoka A, Kondo T, Odaka A et al (1994) Biochemical evidence for the long-tail form (A beta 1-42/43) of amyloid beta protein as a seed molecule in cerebral deposits of Alzheimer’s disease. Biochem Biophys Res Commun 205:834–842
Tomiyama T, Asano S, Furiya Y et al (1994) Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. J Biol Chem 269:10205–10208
Veereshwarayya V, Kumar P, Rosen KM et al (2006) Differential effects of mitochondrial heat-shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem 281:29468–29478
Walsh DM, Klyubin I, Fadeeva JV et al (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Wang R, Sweeney D, Gandy SE et al (1996) The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem 271:31894–31902
Wilhelmus MM, Otte-Holler I, Wesseling P et al (2006) Specific association of small heat-shock proteins with the pathological hallmarks of Alzheimer’s disease brains. Neuropathol Appl Neurobiol 32:119–130
Wilhelmus MM, de Waal RM, Verbeek MM (2007) Heat-shock proteins and amateur chaperones in amyloid-beta accumulation and clearance in Alzheimer’s disease. Mol Neurobiol 35:203–216
Yang T, Hsu C, Liao W et al (2008) Heat-shock protein 70 expression in epilepsy suggests stress rather than protection. Acta Neuropathol 115:219–230
Acknowledgments
This study was supported by Grant-in-Aid CMU97-111 and CMU96-235 from China Medical University, Taichung, Taiwan. We thank Mrs. Shengjie You and Jianliang Zhou for technical assistance and National Cheng Kung University Proteomics Research Core Laboratory for the assistance in mass spectrometry analyses.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, TT., Hsu, CT. & Kuo, YM. Cell-derived soluble oligomers of human amyloid-β peptides disturb cellular homeostasis and induce apoptosis in primary hippocampal neurons. J Neural Transm 116, 1561–1569 (2009). https://doi.org/10.1007/s00702-009-0311-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0311-0