Summary
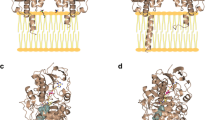
The recent crystallographic structures of human MAO-A and rat MAO-A have shown that human MAO-A is monomeric whereas rat MAO-A is a dimer, even though they share ∼90% sequence identity. The functional significance of this structural difference is unknown. Therefore, biochemical approaches in this paper were performed to investigate the influence of oligomeric state on functional properties of human and rat MAO-A’s. The data show that 1) dimerization of MAO-A increases its structural stability; 2) the differences in kinetic properties may be caused by differences in active site structures as a result of differences in oligomeric states of the human and the rat enzymes; 3) QSAR studies show rat MAO-A as well as human MAO-A catalysis occur via proton abstraction mechanisms, and the binding of substrates is similar for both enzymes.
Similar content being viewed by others
References
AM Andrés M Soldevila A Navarro KK Kidd B Oliva J Bertranpetit (2004) ArticleTitlePositive selection in MAO A gene is human exclusive: determination of the putative amino acid change selected in the human lineage Hum Genet 115 377–386 10.1007/s00439-004-1179-6 Occurrence Handle10.1007/s00439-004-1179-6 Occurrence Handle15349769
A Bondi (1964) ArticleTitleVan der Waals volumes and radii J Phys Chem 68 441–451 Occurrence Handle10.1021/j100785a001 Occurrence Handle1:CAS:528:DyaF2cXls1Cgsg%3D%3D
LD Colibus M Li C Binda A Lustig DE Edmondson A Mattevi (2005) ArticleTitleThree-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B Proc Natl Acad Sci USA 102 12684–12689 10.1073/pnas.0505975102 Occurrence Handle10.1073/pnas.0505975102 Occurrence Handle16129825
C Hansch A Leo D Hoekman (1995) Exploring QSAR SeriesTitleHydrophobic, electronic, and steric constants American Chemical Society Washington, DC
JP Klinman RG Matthews (1985) ArticleTitleCalculation of substrate dissociation constants from steady-state isotope effects in enzyme-catalyzed reactions J Am Chem Soc 107 1058–1060 10.1021/ja00290a052 Occurrence Handle10.1021/ja00290a052 Occurrence Handle1:CAS:528:DyaL2MXnsVOjug%3D%3D
M Li F Hubálek P Newton-Vinson DE Edmondson (2002) ArticleTitleHigh-level expression of human liver monoamine oxidase A in Pichia pastoris: comparison with the enzyme expressed in Saccharomyces cervisiae Protein Expr Purif 24 152–162 10.1006/prep.2001.1546 Occurrence Handle10.1006/prep.2001.1546 Occurrence Handle11812236
J Ma M Yoshimura E Yamashita A Nakagawa A Ito T Tsukihara (2004) ArticleTitleStructure of rat monoamine oxidase A and its specific recognitions for substrates and inhibitors J Mol Biol 338 103–114 10.1016/j.jmb.2004.02.032 Occurrence Handle10.1016/j.jmb.2004.02.032 Occurrence Handle1:CAS:528:DC%2BD2cXisFyks74%3D Occurrence Handle15050826
CM McEwen G Sasaki WR Lenz (1968) ArticleTitleHuman liver mitochondrial monoamine oxidase. I. Kinetic studies of model interactions J Biol Chem 243 5217–5225 Occurrence Handle1:CAS:528:DyaF1cXkvVeltLg%3D Occurrence Handle5702044
CM McEwen G Sasaki DC Jones (1969) ArticleTitleHuman liver mitochondrial monoamine oxidase. II. Determinants of substrate and inhibitor specificities Biochemistry 8 3952–3962 10.1021/bi00838a011 Occurrence Handle10.1021/bi00838a011 Occurrence Handle1:CAS:528:DyaF1MXltF2rsbk%3D Occurrence Handle4899582
JR Miller DE Edmondson (1999) ArticleTitleStructure-activity relationships in the oxidation of para-substituted benzylamine analogues by recombinant human liver monoamine oxidase A Biochemistry 38 13670–13683 10.1021/bi990920y Occurrence Handle10.1021/bi990920y Occurrence Handle1:CAS:528:DyaK1MXmtVOnt7c%3D Occurrence Handle10521274
MC Walker DE Edmondson (1994) ArticleTitleStructure-activity relationships in the oxidation of benzylamine analogues by bovine liver mitochondrial monoamine oxidase B Biochemistry 33 7088–7098 10.1021/bi00189a011 Occurrence Handle10.1021/bi00189a011 Occurrence Handle1:CAS:528:DyaK2cXkslOjtrY%3D Occurrence Handle8003474
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, J., Edmondson, D. Do monomeric vs dimeric forms of MAO-A make a difference? A direct comparison of the catalytic properties of rat and human MAO-A’s. J Neural Transm 114, 721–724 (2007). https://doi.org/10.1007/s00702-007-0678-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-007-0678-8