Abstract
Background
Surgical site infection (SSI) is the most consistently reported complication of cranioplasty. No material showed a categorical superiority in the incidence of infection. Porous polyethylene (PE) is considered a low risk material regarding SSI. However, the literature data are very limited. Thus, our objective was to verify the assumed low incidence of SSI after PE cranioplasty in patients at high risk of SSI. The primary objective was the infection rate, while secondary objectives were implant exposure, revision and cosmetic results.
Method
Patients who underwent three-dimensional (3D) personalized PE cranioplasty in the period 2014–2023 were evaluated prospectively. Only patients with an increased risk of SSI, and a satisfactory clinical conditions were included in the study.
Results
Thirty procedures were performed in 30 patients. Cranioplasty was performed 23 times after hemispheric decompressive craniectomy, five times after limited size craniotomy and two times after bifrontal decompressive craniectomy. Risk factors for the development of infection were 18 previous SSIs, 16 previous repeated revision surgeries, four intraoperatively opened frontal sinuses and two times radiotherapy. Neither infection nor implant exposure was detected in any patient. All patients were satisfied with the aesthetic result. In two cases, a revision was performed due to postoperative epidural hematoma.
Conclusions
Three-dimensional personalized PE cranioplasty is associated with an extremely low incidence of SSI even in high-risk patients. However, our conclusions can only be confirmed in larger studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cranioplasty is a neurosurgical procedure that aims to relieve neurologic symptoms, protect the brain, restore aesthetics, and has a psychosocial impact [4, 7, 10, 22, 25, 43]. In recent years, there has been a resurgence of interest in decompressive craniectomny (DC) with an associated broadening of clinical indications of DC [5, 8, 11, 19, 41]. The frequent use of DC leads to an increased need for a cranioplasty [13]. In addition to the most common reason for cranioplasty, decompressive craniectomy, other indications for cranioplasty are postoperative graft infection, bone flap resorption, bone tumours and post-traumatic and birth calvarial defects [1, 34].
No consensus has yet been established in relation to the optimal material used [12]. Options consist of re-implantation of the autologous bone flap following freezing or subcutaneous preservation, or the use of an alloplastic material. The ideal material for cranioplasty should be biocompatible, radiolucent, non-magnetic, resistant to infections, a non-conductor of heat or cold, porous, malleable, durable, mechanical resistant, readily available, easy to use and low cost [46]. Commonly used commercially available allografts include polymethylmethacrylate (PMMA), titanium, polyetheretherketone (PEEK) and hydroxyapatite (HA).
Despite being technically straightforward, cranioplasty is associated with a high complication rate (15–30%) [45]. The two major sources of failure after cranioplasty are surgical site infection (SSI) and bone flap resorption. While resorption-related cranioplasty failure is associated only with autologous bone, infection can affect any material.
Porous polyethylene (PE) is a relatively innovative material that is not yet widely used, but is nevertheless considered a material with a very low risk of infection [2, 16,17,18, 24, 26, 42]. However, the literature data are very limited.
The aim of our study was to verify the assumed low incidence of SSI after custom-made PE cranioplasty in patients at high risk of SSI. The primary objective was the infection rate, while secondary objectives were implant exposure, revision and cosmetic result.
Methods
A group of 30 patients who underwent cranioplasty with a three-dimensional (3D) personalized PE implant (Su-Por, company Poriferous) in the period 1/2014–3/2023 were prospectively evaluated. Three-dimensional personalized skull implants (PSIs) were only indicated in patients with a satisfactory clinical condition who were at increased risk of SSI. The higher risk of infection was defined using the following risk factors: previous SSI, previous multiple surgeries, intraoperatively opened frontal sinus, and previous radiotherapy.
Our definition of SSI was a persistent or recurrent purulent fistula with an isolated bacterial pathogen. The objectives were assessed using physical examination and contrast computed tomography (CT). The cosmetic result was evaluated by the patients one year after surgery.
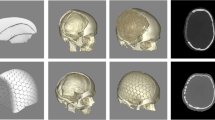
A computer-aided design of a patient-specific porous polyethylene implant was created based on detailed 2 mm bone CT scans. Most designs were based on the shape and curvature of the contralateral side when available. In bifrontal cases, the design engineer collaborated with the neurosurgeon to determine a shape that would work with the patient's anatomy and meet the neurosurgeon's requirements. Bifrontal cases were usually designed in two pieces to facilitate placement (Fig. 1).
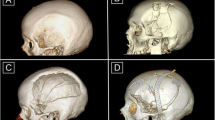
The operation was standardized. The entire head of the patient was preoperatively shaved and bandaged with a disinfectant (peroxyacetic acid) for 12 h. Standard intraoperative antibiotic prophylaxis was administered (Cefazolin). During operations, the patient's head was fixed in a Mayfield fixator. After the scalp incision, the skin flap was separated from the dura mater, duraplasty and bony margins. To prevent epidural hematoma, tack-up sutures were routinely used around the edge of the craniotomy. A custom-made PE implant without flange was then inserted into the bone defect. The implant was fixed with miniplates. In cases of bone flap resorption, the partially resorpted bone flap was removed before insertion of the PE implant. The temporalis muscle was separated from the duraplasty and placed above the implant in an orthotopic position (Fig. 2). A vacuum drain was inserted subgaleally for 24 h. Due to the implant's porous structure providing adequate drainage of the epidural space, additional burholes were not performed in the implant. The galea and skin were sutured.
Skin sutures or staples were removed on postoperative day 14. The first check-up at the outpatient clinic took place two months after the operation, the second 12 months after the operation and then at yearly intervals. A CT scan was performed postoperatively, during the first and second check-ups or when a complication was suspected. Follow-up was at least one year.
Statistical analysis was performed using SW SAS (Cary, NC, USA). The descriptive statistics have been calculated to describe all the investigated variables. The difference in frequencies were tested using Fisher´s exact test. Difference in distributions has been tested using a non-parametric Wilcoxon test. Pearson´s correlation coefficients were used to measure the relationships among examined variables. Statistical significance was determined at the level of 5%.
Results
During the monitored period, 30 cranioplasties were performed in 30 patients. The cohort of patients included 23 men and seven women with an average age of 37 years (range 18–70). All patients were preoperatively in good clinical condition with a Glasgow Coma Scale (GCS) of 15. The primary diagnoses were: 14 traumatic brain injuries (TBI), six tumors (two meningiomas, two gliomas, two calvarial tumors), five intracerebral hemorrhages, three brain abscesses, one cerebral venous sinus thrombosis and one inflammatory demyelinating disease. Cranioplasty was performed 23 times (76,7%) due to a bone defect after hemispheric decompressive craniectomy, five times (16,7%) after limited size craniotomy and twice (6,7%) after bifrontal decompressive craniectomy. Demographic and preoperative data are summarized in Table 1.
The area of the implant was, in 25 cases (83,3%) larger than 100 cm2, and five times (16,7%) the implant was smaller than 100 cm2. Secondary cranioplasty (failure of the previous primary cranioplasty) was performed in 19 patients (63,3%) and primary cranioplasty in 11 patients (36,7%). The timing of cranioplasty was more than three months after the previous surgery in 27 patients (90%) (late cranioplasty) and less than three months after the previous surgery in three patients (10%) (early cranioplasty). The average operative time was 120 min (range 60–210), and the average intraoperative blood loss 241 ml (range 60–885). The average hospital stay was nine days (range 4–14). The price of the implant ranged from 3996 to 9069 Euros (on average 4499 Euros) depending on the size.
There were fourty risk factors found for infection among the 30 patients. Risk factors for infection were as follows: 18 previous SSIs (45%), 16 previous multiple operations (40%), four intraoperatively opened frontal sinuses (10%), two patients were after radiotherapy (5%). Ten patients (33,3%) had a combination of the two risk factors. The following comorbidities were found: smoking in 10 patients (33,3%) and obesity in nine patients (30%). No patient had diabetes mellitus.
The primary objective—the incidence of infection did not occur in any patient. Neither was implant exposure detected in any patient.
In two cases (6,7%), a revision and evacuation of the postoperative epidural hematoma was performed. The cosmetic outcome was satisfactory as expected in all cases. Twenty-six patients (86,7%) were highly satisfied with the aesthetic effect and four patients (13,3%) were only satisfied due to temporal muscle atrophy.
The mean follow-up was 31 months (12–116). No patient was lost to follow-up or excluded from the study. One patient died 5 years after cranioplasty, the cause of death was the progression of an atypical meningioma.
Discussion
Sporadic literature data suggest a low risk of infection in cranioplasty using porous polyethylene. In our study, a highly selective cohort of patients indicated for custom-made cranioplasty with porous polyethylene, who were at an increased risk of infection, was tested. The key finding was the zero incidence of both infection and implant exposure, even in such a high-risk group of patients. Furthermore, the anticipated excellent aesthetic outcomes of 3D cranioplasty were consistently achieved, underscoring the dual benefits of both functional and cosmetic restoration. This study not only fills a gap in the current literature but also provides robust evidence supporting the effectiveness and reliability of porous polyethylene as a preferred material for cranioplasty, particularly in patients with heightened infection risks.
Despite long-lasting debate, no consensus has been reported regarding the best cranioplasty material for cranioplasty [12, 33]. The results of published meta-analyses are controversial, and the strength of the analyses was additionally limited by the low quality and heterogeneity of the literature [3, 9, 12, 21, 23, 27, 40, 45]. Some meta-analyses have demonstrated the convincing inferiority of autologous bone. Others have shown that autologous bone can still be the first choice in patients with a low risk of resorption or for whom resorption might not be of major concern [3, 9, 21, 23, 40]. Aseptic bone flap resorption is unique to autologous bone and is the reason for a significantly higher removal rate and probably the decline in autologous cranioplasty. Discontinuance of institutional tissue banking due to increasing storage costs or national regulation with strict mandated biobanking requirements may also be reasons for switching to alloplastic materials in some countries [20, 37, 40, 46]. Moreover, the choice of material is not only addressed from the point of view of the incidence of surgical complications, but also from the health-economic perspective and established practices in different countries. As reported by Morselli et al., titanium is mostly used in Australia, the United Kingdom and Germany, PMMA in the USA, PEEK in the USA, Singapore and South Korea and HA in France and Italy [27].
All materials are now being deployed to produce 3D personalized skull implants [44]. Although PSIs improve the cosmetic benefit, they have not yet been proven to have a positive impact on clinical outcome [12]. Recent advances in 3D printing technology, enabling in-house manufacturing of PSIs and bypassing medical firms, will lead to lower prices and shift the cost-effectiveness balance more towards allogenic materials [15, 31, 38]. Nevertheless, autologous bone is still the most commonly used material due to its high degree of biocompatibility, low cost, ease of acquisition, good fit and contour, zero risk of disease transmission, and because it is viable [12, 14].
Surgical site infection is the most consistently reported complication of cranioplasty with an infection rate of 5.9% to 8,6% [23, 40, 45]. No material showed a categorical superiority in the incidence of infection. However, a standardized definition of postcranioplasty infection is lacking, which further reduces the comparability of studies. Analysis of the best current evidence in published meta-analyses suggests that neither implant material nor early surgery (up to three months) has any effect on the rate of infection after cranioplasty [3, 9, 21, 23, 40, 45]. Most graft infection occurred less than three months after cranioplasty and infection discovered more than one year after cranioplasty is rarely seen [39]. Therefore, a post-cranioplasty follow-up of at least 12 months was chosen for our study.
Porous polyethylene is an innovative bioinert polymer, which has also been investigated for cranial implants [35]. PE combines a porous structure and a smooth membrane. The fibrous side promotes fibrovascular and bone ingrowth. As reported by Gosain et al., PE facilitates vascular and soft-tissue in-growth into its pores by week one, and bone ingrowth by three weeks [6]. Fibrovascular integration into pores should reduce the risk of infection. Porous polyethylene is considered to be a material with a very low risk of infection. However, the literature data are limited and conclusive evidence is lacking.
Mostly only small retrospective studies have been published. Mokal et al. and Kumar et al. reported no infection after PE cranioplasty in seven and five patients respectively [18, 26]. Wang et al. detected only one infection in a cohort of 23 patients [42]. Rare complications after PE cranioplasty in 12 patients in comparison with 32 PMMA cranioplasties were presented by Celik et al. [2]. Marlier et al. confirm that PE is an excellent restorative material for the cranial defect after decompressive craniectomy. There was only one scalp necrosis with infection in a group of 23 patients [24]. Konofaos et al. evaluated 18 patients after PE implant reconstruction of large craniofacial defects. Implant exposure was observed in three patients, and of these one required removal of the implant [17]. The largest cohort of 32 patients after PE cranioplasty with an incidence of infection of 6,3% was presented by Kim et al. [16].
The advantage of polyethylene is the possibility of using a prefabricated form which, due to its consistency, can be easily adjusted and modeled during the operation. Prefabricated implants are mostly used for small defects, or in patients for whom the aesthetic outcome might not be of major concern. Of course, the 3D personalized form is more sophisticated and aesthetically perfect.
The price of polyethylene is acceptable. With the exception of PMMA, the price of PE is the lowest among the alloplastic materials used for PSI (Titan, PEEK, HA) [40].
At our department, the management of cranioplasty has gradually changed over time. Until 2015, an autologous bone flap sterilized in an autoclave was used for cranioplasty. It was accompanied by a low risk of infection (3,3%), but a disproportionately high incidence of resorption (20%) [28]. Sterilization of autologous bone by autoclaving was therefore abandoned and replaced by extra-corporeal cryopreservation. In 2014, we started using porous polyethylene for patients with an increased risk of infection [32]. PSI implants are only indicated for patients with a satisfactory clinical condition and a good prognosis, for whom the PSIs are covered by general health insurance in our country. Thus, all patients included in this prospective study were at risk of developing SSI and had a satisfactory clinical condition and a good prognosis.
We expected that the incidence of infection in our study would be low, but the zero incidence surprised us. However, our results are not statistically significant compared to the mean infection rate of 7.25% (5.9%—8.6%) reported in the literature (p = 0,169; 95% CI 0,0000 to 0,1157) [45]. The reason is the insufficient number of patients in our cohort. Through simulation, we found that a statistically significant difference would be achieved in a group of 45 patients with zero incidence of infection (95% CI 0,0000 to 0,0787). On the other hand, in our study, PE was used exclusively in patients with an increased risk of infection in contrast to published literature data.
The most significant risk factors for infection are, demonstrably, multiple surgeries and previous infections [30]. In our study, the increased risk of infection was defined using the risk factors. The most common risk factor was infection of a previous primary cranioplasty with subsequent removal of a bone flap or implant (18 times), as well as previous multiple operations (16 times). In four patients, the frontal sinus was opened during the operation. Of these, in two cases, the sinuses were opened widely and bilaterally during bifrontal decompressive craniectomy. In the other two cases, the frontal sinus was opened during the resection of a calvarial tumor extending into the frontal sinus. In these cases, cranioplasty with porous polyethylene was performed primarily, at the same time as the tumor resection and sinus plasty. In two patients, cranioplasty was performed in patients who underwent radiotherapy after low-grade astrocytoma resection. Ten patients even had a combination of two risk factors. Common comorbidities such as smoking, diabetes, and obesity, can also contribute to the incidence of infection. While diabetes had never been diagnosed for any of the patients and therefore did not affect the incidence of infection, the relatively high proportion of smokers (33%) and obese people (30%) may have contributed to the increased risk of infection. On the other hand, the good general condition of the patients and their young age may have contributed to the negligible incidence of infection.
The local application of antibiotics could not influence the SSI, as neither the implant antibiotic solution nor the intrawound application of antibiotic powder was used in any patient. In all cases, only standard intravenous intraoperative antibiotic prophylaxis was used.
Although wound drainage is generally considered a risk factor for infection, Spake et al. showed that subgaleal drains are associated with reduced infection rates after autologous cranioplasty [36]. In our study, a closed vacuum suction drain was used in all patients. The drain was always extracted on the first postoperative day. Therefore, we do not consider drainage to be a risk factor for infection in our group, but rather a factor that could have prevented it.
Exposure of the implant, which can be associated with infection, was not observed in any of our patients. The average incidence of implant exposure is given in the literature as 6% [9]. The risk of exposure in PSIs may also be related to suture under tension due to scalp atrophy. In patients with less significant scalp atrophy, loosening the skin edges or reducing the implant size is sufficient. The overall shape of the PSI can be adjusted as needed preoperatively during the manufacturing process. For example, more convexity may be requested if there is temporal hollowing, while less convexity may be requested if the patient does not have enough soft tissue to cover the implant. Although the nature of PE allows for it, the implants were not scaled down in any of our patients to prevent undue pressure on the skin. However, in cases of significant shrinkage of the skin, the implantation of subcutaneous expanders is necessary. In our study, soft tissue expansion before delayed cranioplasty was performed in two patients [29].
The average incidence of revision after cranioplasty is reported to be 14% [9]. PEEK had the lowest risk of re-operation with a rate of 5% [9]. The highest revision rate, significantly related to resorption, is described in autografts (18%) [9]. Revision was necessary in two of our patients (6.7%). In both cases, it was for cranioplasty after hemispheric decompressive craniectomy and the reason was epidural hematoma. Both patients fully recovered after evacuation of the hematoma. The cause of the epidural hematomas was not convincingly identified in either case. Standard preventive measures were used for all patients—tack-up sutures around the craniotomy and subcutaneous vacuum drainage. The porous structure of the implant also allowed for drainage of the epidural space. Both patients were obese non-smokers. The surgery in both cases was accompanied by more complex hemostasis, which corresponded with the above-average blood loss. However, both patients had normal hemostatic parameters. We suspect that the development of epidural hematomas was likely related to insufficient hemostasis.
The last secondary objective was cosmetic result. Considering the PSI results, it was no surprise that all patients were satisfied with the aesthetic result. Only four patients did not rate the aesthetic result as highly satisfactory due to temporal muscle atrophy. In all four cases, it was cranioplasty after hemispheric decompressive craniectomy. Both patient and clinician satisfaction showed we were in agreement.
Our manuscript presents the second largest cohort of patients after cranioplasty with porous polyethylene and is the only published prospective study to date. In none of the published studies was PE cranioplasty exposed to such a systematic risk of infection as in our group. In addition, most of our cases (24 patients) involved cranioplasty of large calvarial defects after decompressive craniectomies.
One of the goals of our article was to draw attention to porous polyethylene, which is a surprisingly neglected material in the field of cranioplasty, and at least to partially fill a gap in the literature.
The limitations of our study are the heterogeneity of the group of patients with different primary pathologies and different sizes of cranioplasty and the relatively long time period of patient recruitment.
Conclusion
Our results show that custom-made cranioplasty with porous polyethylene in patients with an increased risk of infection is accompanied by minimal morbidity. No infection or wound dehiscence with implant exposure was noted, and revision surgery was rare. As expected, all patients were satisfied with the aesthetic effect of PSIs cranioplasty. Porous polyethylene is a very effective and reliable allogeneic material for performing cranioplasty and it deserves to be more widespread not only in patients with an increased risk of infection. Only further clinical studies with better evidence could confirm our conclusions.
Data availability
The data that formed the basis of the study and that support our results can be obtained from the corresponding author upon reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DC:
-
Decompressive craniectomy
- GCS:
-
Glasgow Coma Scale
- HA:
-
Hydroxyapatite
- PE:
-
Porous polyethylene
- PEEK:
-
Polyetheretherketone
- PMMA:
-
Polymethylmethacrylate
- PSIs:
-
Personalized skull implants
- SSI:
-
Surgical site infection
- TBI:
-
Traumatic brain injury
- 3D:
-
Three-dimensional
References
Alkhaibary A, Alharbi A, Alnefaie N, Oqalaa Almubarak A, Aloraidi A, Khairy S (2020) Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg 139:445–452
Celik H, Kurtulus A, Yildirim ME, Tekiner A, Erdem Y, Kantarci K, Kul H, Bayar MA (2022) The comparison of autologous bone, methyl-methacrylate, porous polyethylene, and titanium mesh in cranioplasty. Turk Neurosurg 32(5):841–844
Cerveau T, Rossmann T, Clusmann H, Veldeman M (2023) Infection-related failure of autologous versus allogenic cranioplasty after decompressive hemicraniectomy - A systematic review and meta-analysis. Brain Spine 3:101760
Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M (1997) Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res 19(3):311–316
Fung C, Murek M, Klinger-Gratz PP, Fiechter M, Z’Graggen WJ, Gautschi OP, El-Koussy M, Gralla J, Schaller K, Zbinden M, Arnold M, Fischer U, Mattle HP, Raabe A, Beck J (2016) Effect of decompressive craniectomy on perihematomal edema in patients with intracerebral hemorrhage. PLoS One 11(2):e0149169
Gosain AK, Persing JA (1999) Biomaterials in the face: benefits and risks. J Craniofac Surg 10(5):404–414
Halani SH, Chu JK, Malcolm JG, Rindler RS, Allen JW, Grossberg JA, Pradilla G, Ahmad FU (2017) Effects of cranioplasty on cerebral blood flow following decompressive craniectomy: A systematic review of the literature. Neurosurgery 81(2):204–216
Hawryluk GWJ, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J (2020) Guidelines for the management of severe traumatic brain injury: 2020 Update of the decompressive craniectomy recommendations. J Neurosurgery 87(3):427–434
Henry J, Amoo M, Taylor J, O’Brien DP (2021) Complications of cranioplasty in relation to material: Systematic Review, Network Meta-Analysis and Meta-Regression. Neurosurgery 89(3):383–394
Honeybul S, Janzen C, Kruger K, Ho KM (2013) The impact of cranioplasty on neurological function. Br J Neurosurg 27(5):636–641
Hutchinson PJ, Kolias AG, Tajsic T, Adeleye A, Aklilu AT, Apriawan T, Bajamal AH, Barthélemy EJ, Devi BI, Bhat D, Bulters D, Chesnut R, Citerio G, Cooper DJ, Czosnyka M, Edem I, El-Ghandour NMF, Figaji A, Fountas KN, Gallagher C, Hawryluk GWJ, Iaccarino C, Joseph M, Khan T, Laeke T, Levchenko O, Liu B, Liu W, Maas A, Manley GT, Manson P, Mazzeo AT, Menon DK, Michael DB, Muehlschlegel S, Okonkwo DO, Park KB, Rosenfeld JV, Rosseau G, Rubiano AM, Shabani HK, Stocchetti N, Timmons SD, Timofeev I, Uff C, Ullman JS, Valadka A, Waran V, Wells A, Wilson MH, Servadei F (2019) Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury : Consensus statement. Acta Neurochir (Wien) 161(7):1261–1274
Iaccarino C, Kolias A, Adelson PD, Rubiano AM, Viaroli E, Buki A, Cinalli G, Fountas K, Khan T, Signoretti S, Waran V, Adeleye AO, Amorim R, Bertuccio A, Cama A, Chesnut RM, De Bonis P, Estraneo A, Figaji A, Florian SI, Formisano R, Frassanito P, Gatos C, Germanò A, Giussani C, Hossain I, Kasprzak P, La Porta F, Lindner D, Maas AIR, Paiva W, Palma P, Park KB, Peretta P, Pompucci A, Posti J, Sengupta SK, Sinha A, Sinha V, Stefini R, Talamonti G, Tasiou A, Zona G, Zucchelli M, Hutchinson PJ, Servadei F (2021) Consensus statement from the international consensus meeting on post-traumatic cranioplasty. Acta Neurochir (Wien) 163(2):423–440
Iaccarino C, Kolias AG, Roumy LG, Fountas K, Adeleye AO (2020) Cranioplasty following decompressive craniectomy. Front Neurol 10:1357
Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N (2003) The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery 52(3):591–596
Kim BJ, Hong KS, Park KJ, Park DH, Chung YG, Kang SH (2012) Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J Korean Neurosurg Soc 52(6):541–546
Kim JK, Lee SB, Yang SY (2018) Cranioplasty using autologous bone versus porous polyethylene versus custom-made titanium mesh: A retrospective review of 108 patients. J Korean Neurosurg Soc 61(6):737–746
Konofaos P, Thompson RH, Wallace RD (2017) Long-term outcomes with porous polyethylene implant reconstruction of large craniofacial defects. Ann Plast Surg 79(5):467–472
Kumar NG, Sreenivas M, Gowda S (2016) Cranioplasty of large cranial defects with porous polyethylene implants. J Craniofac Surg 27(4):e333–e335
Lin J, Frontera JA (2021) Decompressive hemicraniectomy for large hemispheric strokes. Stroke 52(4):1500–1510
Lindner D, Schlothofer-Schumann K, Kern BC, Marx O, Müns A, Meixensberger J (2017) Cranioplasty using custom-made hydroxyapatite versus titanium: a randomized clinical trial. J Neurosurg 126(1):175–183
Liu L, Lu ST, Liu AH, Hou WB, Cao WR, Zhou C, Yin YX, Yuan KS, Liu HJ, Zhang MG, Zhang HJ (2020) Comparison of complications in cranioplasty with various materials: a systematic review and meta-analysis. Br J Neurosurg 34(4):388–396
Mah JK, Kass RA (2016) The impact of cranioplasty on cerebral blood flow and its correlation with clinical outcome in patients underwent decompressive craniectomy. Asian J Neurosurg 11(1):15–21
Malcolm JG, Mahmooth Z, Rindler RS (2018) Autologous cranioplasty is associated with increased reoperation rate: A systematic review and meta-analysis. World Neurosurg 116:60–68
Marlier B, Kleiber J-C, Bannwarth M, Theret E, Eap C, Litre CF (2017) Reconstruction of cranioplasty using medpor porouspolyethylene implant. Neurochirurgie 63(6):468–472
Mee H, Anwar F, Timofeev I, Owens N, Grieve K, Whiting G, Alexander K, Kendrick K, Helmy A, Hutchinson P, Kolias A (2022) Cranioplasty: A multidisciplinary approach. Front Surg 9:864385
Mokal NJ, Desai MF (2011) Calvarial reconstruction using high-density porous polyethylene cranial hemispheres. Indian J Plast Surg 44(3):422–431
Morselli C, Zaed I, Tropeano MP, Cataletti G, Iaccarino C, Rossini Z, Servadei F (2019) Comparison between the different types of heterologous materials used in cranioplasty: a systematic review of the literature. J Neurosurg Sci 63(6):723–736
Mracek J, Hommerova J, Mork J, Richtr P, Priban V (2015) Complications of cranioplasty using a bone flap sterilised by autoclaving following decompressive craniectomy. Acta Neurochir (Wien) 157(3):501–506
Mracek J, Richtr P, Seidl M, Dostal J, Tupy R, Priban V (2022) Staged scalp soft tissue expansion before CAD/CAM porous polyethylene cranioplasty. Cesk Slov Neurol N 85/118(1):1–4
Paredes I, Castaño-León AM, Munarriz PM, Martínez-Perez R, Cepeda S, Sanz R, Alén JF, Lagares A (2015) Cranioplasty after decompressive craniectomy. A prospective series analyzing complications and clinical improvement. A. Neurocirugia (Astur) 26(3):115–25
Park EK, Lim JY, Yun IS, Kim JS, Woo SH, Kim DS, Shim KW (2016) Cranioplasty enhanced by three-dimensional printing: Custom-made three-dimensional-printed titanium implants for skull defects. J Craniofac Surg 27(4):943–949
Seidl M, Mracek J, Dostal J, Priban V (2022) Computer-modeled cranioplasty from porous polyethylene in high-risk terrain. Cesk Slov Neurol N 85/118(5):410–413
Servadei F, Iaccarino C (2015) The therapeutic cranioplasty still needs an ideal material and surgical timing. World Neurosurg 83(2):133–135
Shah AM, Jung H, Skirboll S (2014) Materials used in cranioplasty: a history and analysis. Neurosurg Focus 36(4):E19
Siracusa V, Maimone G, Antonelli V (2021) State-of-art of standard and innovative materials used in cranioplasty. Polymers (Basel) 13(9):1452
Spake CSL, Beqiri D, Rao V, Crozier JW, Svokos KA, Woo AS (2021) Subgaleal drains may be associated with decreased infection following autologous cranioplasty: a retrospective analysis. Br J Neurosurg 9:1–7
Stefini R, Esposito G, Zanotti E, Iaccarino C, Fontanella MM, Servadei F (2013) Use of “custom made” porous hydroxyapatite implants for cranioplasty: postoperative analysis of complications in 1549 patients. Surg Neurol Int 4:12
Tan ET, Ling JM, Dinesh SK (2016) The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J Neurosurg 124(5):1531–1537
Tokoro K, Chiba Y, Tsubone K (1989) Late infection after cranioplasty–review of 14 cases. Neurol Med Chir (Tokyo) 29(3):196–201
van de Vijfeijken SECM, Münker TJAG, Spijker R, Karssemakers LHE, Vandertop WP, Becking AG, Ubbink (2018) Autologous bone is inferior to alloplastic cranioplasties: Safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg 117:443-452.e8
Veldeman M, Weiss M, Daleiden L, Albanna W, Schulze-Steinen H, Nikoubashman O, Clusmann H, Hoellig A, Schubert GA (2022) Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage-justifiable in light of long-term outcome? Acta Neurochir (Wien) 164(7):1815–1826
Wang JC, Wei L, Xu J, Liu JF, Gui L (2012) Clinical outcome of cranioplasty with high-density porous polyethylene. J Craniofac Surg 23(5):1404–1406
Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K (2000) Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg 93(1):53–61
Wolff A, Santiago GF, Belzberg M, Huggins CH, Lim M, Weingart J, Anderson W, Coon A, Huang J, Brem H, Gordon Ch (2018) Adult cranioplasty reconstruction with customized cranial implants: Preferred technique, timing, and biomaterials. J Craniofac Surg 29(4):887–894
Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD (2011) Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery 68(4):1124–1129
Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC (2016) Cranioplasty: Review of materials. J Craniofac Surg 27(8):2061–2072
Funding
This study was supported by the grant of Ministry of Health of the Czech Republic – Conceptual Development of Research Organization (Faculty Hospital in Pilsen – FNPl, 00669806).
Author information
Authors and Affiliations
Contributions
Jan Mracek, Miroslav Seidl and Vladimir Priban wrote the main manuscript. Jiri Dostal and Petr Kasik processed the database. Radek Tupy evaluated CT. Irena Holeckova neurologically examined the patients. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mracek, J., Seidl, M., Dostal, J. et al. Three-dimensional personalized porous polyethylen cranioplasty in patients at increased risk of surgical site infection. Acta Neurochir 166, 383 (2024). https://doi.org/10.1007/s00701-024-06281-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06281-x