Abstract
Background
Ventriculostomy-associated infection (VAI) is common after external ventricular drains (EVD) insertion but is difficult to diagnose in patients with acute brain injury. Previously, we proposed a set of criteria for ruling out VAI in traumatic brain injury. This study aimed to validate these criteria. For exploratory purposes, we sought to develop and validate a score for VAI risk assessment in patients with different types of severe acute brain injury.
Methods
This retrospective cohort study included adults with acute brain injury who received an EVD and in whom CSF samples were taken over a period of 57 months. As standard non-coated bolt-connected EVDs were used. The predictive performance of biomarkers was analyzed as defined previously. A multivariable regression model was performed with five variables.
Results
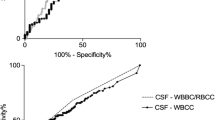
A total of 683 patients with acute brain injury underwent EVD placement and had 1272 CSF samples; 92 (13.5%) patients were categorized as culture-positive VAI, 130 (19%) as culture-negative VAI, and 461 (67.5%) as no VAI. A low CSF WBC/RBC ratio (< 0.037), high CSF/plasma glucose ratio (> 0.6), and low CSF protein (< 0.5g/L) showed a positive predictive value of 0.09 (95%CI, 0.05–0.13). In the multivariable logistic regression model, days to sample (OR 1.09; 95%CI, 1.03–1.16) and CSF WBC/RBC ratio (OR 34.86; 95%CI, 3.94–683.15) were found to predict VAI.
Conclusion
In patients with acute brain injury and an EVD, our proposed combined cut-off for ruling out VAI performed satisfactorily. Days to sample and CSF WBC/RBC ratio were found independent predictors for VAI in the multivariable logistic regression model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with acute brain injury, such as hemorrhagic stroke and traumatic brain injury (TBI), external ventricular drains (EVDs) are frequently used to divert cerebrospinal fluid (CSF) and monitor intracranial pressure (ICP) [1]. The most common complication to EVD treatment is ventriculostomy-associated infection (VAI) [2], which frequently is due to the easier access for microorganisms to the intrathecal space. Patients with VAI are at risk of prolonged duration of hospital stay and EVD treatment as well as a need for permanent CSF drainage device [3]. Previous studies have found several risk factors associated to VAI (Supplemental material), but still the reported prevalence of VAI varies in the literature from 6 [4] to 36% [5] of all patients with an EVD; two meta-analyses have suggested prevalence’s of 11% [3] and 23% [6], respectively. This variation is probably due to the lack of standardized diagnostic criteria [7, 8].
The diagnostic challenge
Typical clinical signs and symptoms associated with VAI are fever, headache, neck stiffness, cranial nerve deficits, seizures, and altered mental state [6, 8]. These symptoms are often difficult to distinguish from the clinical presentation in uninfected patients with acute brain injury, due to the damage or inflammation caused by the brain damage [9]. Patients that receive EVDs are typical critically ill and furthermore, often sedated and may have extracranial infection, such as ventilator-associated pneumonia, urinary tract infection or catheter-related bloodstream infection [10, 11]. All these factors render VAI difficult to diagnose accurately from clinical and paraclinical examinations. The currently most important test for diagnosing VAI therefore is the detection of microorganisms cultured from CSF, which, however, may lead to a delay of up to 10 days before a definitive answer is provided [12]. Moreover, this test does not in itself distinguish between contamination, colonization, and infection [6, 8]. Therefore, there is a need for faster and more accurate diagnostics. Previously, Willer-Hansen et al. proposed three criteria of CSF WBC/RBC ratio < 0.037, low CSF protein < 0.5g/L, and high CSF/P glucose ratio > 60%, which, when combined, appear to have a positive predictive value (PPV) at 0 (95% confidence interval (CI), 0.0–0.14) for VAI in patients with TBI, thereby effectively ruling out VAI [13].
The aim of this study was to validate the previously proposed cut-off for ruling out VAI in a broader population of patients with acute brain injury, in whom an EVD has been inserted [13]. Furthermore, we exploratory aimed to develop a score for estimating the risk of VAI, using a combination of readily available biomarkers to guide clinicians in their decision on whether to begin treatment.
Methods
Medical records were retrospectively screened for patients ≥ 18 years with acute brain injury, in whom an EVD was inserted at the Department of Neurosurgery, Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark, between 6 November 2016 and 25 July 2021. The study was approved by the Directors of the Neuroscience Centre and the Departments of Neurosurgery and Neuroanaesthesiology, respectively, at Rigshospitalet (protocol id: 20069795), and approved as a register study from Centre of Regional Development (file number: R-21047752). According to Danish law, no other approval was necessary.
Acute brain injury was defined as TBI, spontaneous intracerebral hemorrhage (ICH), or non-traumatic subarachnoid hemorrhage (SAH). Non-traumatic SAH was divided by the underlying cause, i.e., an aneurismal bleed or other causes (such as, but not restricted to, an arteriovenous malformation). Patients with pre-existing cerebral infection (e.g., meningitis, cerebral abscess) or having ventriculoperitoneal (VP) or ventriculoatrial (VA) shunts, tumors, ischemic apoplexies, and patients in whom an EVD was placed electively for diagnostic purposes, were excluded.
EVDs were inserted under sterile conditions in the operating room. As standard non-coated, bolt-connected EVDs were used. If an EVD had to be replaced the procedure was done either in the operating room or at the intensive care unit. During the admission, CSF samples were collected under sterile conditions at the discretion of the attending clinician. Samples were sent to the Department of Clinical Biochemistry for biochemical analyses and to Department of Clinical Microbiology for microscopy, culturing, and antibiotic susceptibility testings.
Data collection
From medical records, the following was registered: age. gender, time of ictus, diagnosis, characteristics of EVD (placement, localization, replacement, and removal), neurosurgical or endovascular procedures, treatment characteristics (antibiotic treatment and treatment length), survival status, biochemical laboratory parameters (plasma and CSF), and microbiological data. CSF and plasma samples were collected on the same day.
Terminology
The patients were classified as follows (Figure 1): culture-positive VAI for patients treated for VAI that had a positive CSF culture, which was not attributed to contamination; culture-negative VAI for patients treated with antibiotics for suspected VAI but with negative CSF culture; and no VAI for patients never treated for VAI and who had negative CSF cultures throughout the EVD treatment period.
CSF samples were categorized as negative, pre-positive, or positive. A positive sample corresponded to the patient’s first sample that was culture-positive, while pre-positive was used for samples drawn before the positive sample in these patients (Supplemental material). Pre-positive samples and samples collected after a positive sample were excluded. A negative sample was used for samples in patients with no VAI, and for all samples drawn prior to antibiotic treatment targeting VAI in patients with culture-negative VAI (Table 2).
Nomenclature
Willer-Hansen et al. used the term “ventriculostomy-related infection (VRI),” for the sake of clarity, we decided consistently to use the term “ventriculostomy-associated infection (VAI)” to refer to the same concept, in alignment with their publication. The same applies to other synonymous terms representing EVD-related infections.
Outcomes
The primary outcome was the performance of the proposed cut-off for ruling out VAI by Willer-Hansen et al. [13]. We used the same definitions of pleocytosis (CSF WBC/RBC ratio > 0.037), hypoglycorrhachia (CSF/plasma glucose ratio < 0.6 [14]), and increased CSF protein (> 0.5g/L) and calculated the sensitivity, specificity, positive predictive-, and negative predictive values. For this outcome, CSF samples were classified as either positive or negative, thereby excluding the pre-positive samples.
Data handling and statistical analyses
Data were stored in REDCap (REDCap version 12.0.3 Vanderbilt University, Tennessee, United States of America). Statistical analyses were carried out using R (R version 4.2.2, R Core Team 2022, Vienna, Austria). Continuous variables were tested for normality using Shapiro-Wilk test for normality. Normally distributed data are presented using mean and standard deviations (SD), while non-normally distributed data are presented using the median and interquartile range or 1st or 3rd quartile. Categorical variables are presented as counts and percentages. p < 0.05 was considered statistically significant.
For validation of the tree combined criteria for ruling out VAI, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for both individual and combined criteria.
A multivariable logistic regression was performed to analyze the association between positive and negative CSF culture and five variables with potential association to VAI [6, 15,16,17,18,19,20,21,22,23]: number of days with EVD, CSF WBC/RBC ratio, CSF/plasma glucose ratio, CSF neutrophil granulocyte (NG)/WBC ratio, and CSF protein (Table 5).
As an exploratory outcome, we intended to evaluate the performance of a machine learning model as derived in a development cohort and tested in a validation cohort. It has since become apparent that decisions regarding the design probably contributed to the poor performance of, and introduced limitations, to the model. In the interest of full disclosure, methods and results regarding the model are described in full in the Supplemental material.
Results
From 6 November 2016 to 25 July 2021, we identified 683 patients with acute brain injury who were treated with an EVD and had at least one CSF sample drawn (Figure 1). Ninety-two (13.5%) patients were classified as culture-positive VAI, 130 (19%) patients as culture-negative VAI, and 461 (67.5%) patients as no VAI (Table 1). The number of EVD placements was highest among patients with acute brain injury in 2017, coinciding with the peak occurrence of culture-positive VAI cases (Supplemental material). The distribution between No VAI, culture-negative VAI, and culture-positive VAI varied between different types of acute brain injury (Supplemental material)
Patients with culture-positive VAI had their EVD in situ for longer time, more frequently had bilateral EVDs, and were more likely to experience EVD replacement than patients with no VAI. Intrathecal (IT) antibiotic treatment was given to almost all patients with culture-negative VAI or culture-positive VAI in the form of vancomycin 20 mg once daily. Patients with culture-positive VAI received treatment for a median of 7 days (Q1; Q3: 5; 10), and patients with culture-negative VAI for 6 days (Q1; Q3: 4; 9) days (Fig. 2, Table 1).
Timeline. Day 0 refers to the day of the EVD placement. EVD, all patients included in the study; EVD removed, patients who had their EVD removed; VP shunt, patients who had their EVD replaced by a VP shunt; died, patients who died; culture positive VAI, patients treated for VAI with a culture-positive sample; culture negative VAI, patients who were treated for VAI at the clinicians’ discretion, but with culture-negative samples
CSF samples
In total, 1272 CSF samples were drawn for microbial culture and biochemical analysis (Table 2). The median number of samples per patient was 2 (Q1; Q3: 1; 3). The positive samples had overall higher values of CSF-WBC, CSF-WBC/RBC, CSF NG, and CSF NG/WBC than the negative samples, whereas the CSF/plasma glucose ratio was lower in the positive samples (Table 2). Eighty-nine CSF cultures were culture-positive, of which 89% yielded Gram-positive bacteria (most commonly Staphylococcus epidermidis) and 8% Gram-negative bacteria; fungi were present in 3% (Table 3).
Ruling out VAI
For ruling out VAI, the combined cut-off suggested by Willer et al. [13], 9% of the patients had a positive sample, with a low CSF WBC/RBC ratio (< 0.037), high CSF/plasma glucose ratio (> 0.6), and low CSF protein (< 0.5g/L), whereas 91% had a negative sample (PPV 0.09; 95%CI, 0.05–0.13) (Table 4).
Multivariable logistic regression
The multiple logistic regression analysis showed a significant increase in the odds of having a positive CSF culture associated with a higher number of days to sample (OR, 1.09; 95%CI, 1.03–1.16) and increased CSF WBC/RBC ratio (OR 34.86, 95%CI 3.94–683.15). Additionally, the CSF/plasma glucose ratio (OR, 0.73; 95%CI 0.09–5.73), CSF protein (OR, 1.21; 95%CI 0.71–1.88), and CSF NG/WBC ratio (OR 2.74, 95%CI 0.78–9.57) were not found as significant predictors for a positive CSF culture (Table 5).
VAI prediction score
The VAI prediction score was derived in the development cohort, in which it showed moderate accuracy (area under the curve (AUC) 0.69; 95%CI, 0.62–0.77) (Supplemental material). In the validation cohort, it performed no better than chance (AUC 0.46; 95%CI, 0.30–0.79). Similarly, Youden’s threshold showed moderate accuracy (AUC 0.69; 95%CI, 0.61–0.77) in the development cohort, and low accuracy in the validation cohort (AUC 0.55; 95%CI, 0.30–0.79).
Discussion
In this retrospective study of 683 patients with acute brain injury, we found a PPV for having a positive sample of 9%, when using the combined cut-off for ruling out VAI identified by Willer et al. [13].
In our cohort, patients with culture-positive VAI had their EVD for longer time than patients with no VAI. Although prolonged EVD treatment is an accepted risk factor for VAI [8, 15, 24,25,26], it may also be a consequence of the time waiting for antibiotic treatment effect and repeatedly negative CSF cultures in patients needing replacement of the EVD with a permanent internal shunt. Also, culture-positive VAI patients were more likely to undergo EVD replacement, compared to no VAI patients, which could be attributed to potential dysfunction or leak from the EVD, or a decision to treat VAI with intraventricular antibiotics. Our cohort resembled those in previous reports with regard to culture-positive rate and the biochemical patterns of neutrophil pleocytosis and hypoglycorrhachia [6, 13, 15, 17, 21, 27]. Unfortunately, we were unable to test the added diagnostic benefit of CSF lactate and Gram staining in our cohort as CSF lactate is not measured routinely in our department, and the CSF Gram stain was difficult to retrieve due to the retrospective design.
Several preventive measures have been explored in prior studies to reduce the incidence of VAI. These include utilization of EVD catheters coated with antimicrobial materials [28, 29], prophylactic administration of antibiotics [30], minimizing the frequency of CSF samples [31], and fixation to the skin with either tunneled or bolted EVDs [32]. Notwithstanding these efforts, in patients in whom the clinician suspects VAI, distinguishing between the inflammatory response arising from acute brain injury and infection remains challenging [9]. Koopman et al. investigated the time course of simple CSF variables for 20 days in patients with aSAH. The authors reported a significant increase in CSF leucocyte count six days post-ictus, accompanied by a decrease in RBC count; moreover, CSF protein stayed elevated 20 days after ictus [33]. Together, these findings emphasize the need for better multivariable diagnostic tools. Muñoz-Gómez et al. tested a combination of CSF lactate > 6 nmol/L, CSF pleocytosis (> 50 WBC/mm3), a positive CSF Gram stain, and a positive CSF culture of neuropathogens (with the same morphology) [34], although this did not yield to any false-positive results in their validation; only 22 patients were studied, of whom only one had VAI. Consequently, while this combination might be of interest, it does not avoid the time-consuming process of CSF culturing. Boeer et al. proposed a predictive model for positive CSF cultures, using a classification and regression trees analysis. The combination of CSF IL-6, blood leukocyte count, and plasma C-reactive protein, yielded an area under the receiver-operating characteristic curve of 0.89 (no CI), with a high sensitivity, specificity, and NPV [35]. Others have also tested diagnostic criteria for postoperative meningitis, but these studies were not limited to patients with EVDs and acute brain injury [18, 36].
Predictors for VAI
According to the multivariable logistic regression, days to sample and CSF WBC/RBC ratio were independent predictors for a positive CSF culture, whereas CSF/plasma glucose ratio, CSF protein, and CSF NG/WBC ratio were not associated with a positive CSF culture. These findings align with the 2017 recommendations of the Infectious Diseases Society of America, underscoring the limitations of relying solely on abnormalities in CSF cell count, glucose, and protein as reliable indicators for the presence of infection [37]. However, several studies have found associations between these markers and VAI in univariate analyses [6, 15,16,17, 19,20,21, 23].
Diagnostic challenges
As touched upon above, the clinical diagnosis of intracranial infection, and in particular VAI, is notoriously difficult in patients with acute brain injury because of pre-existing sedation or impaired consciousness, intracranial inflammation, and variable levels of intracranial bleeding. CSF culture is a slow procedure and may also be unreliable because the frequent use of systemic antibiotics may produce false-negative results on one side, whereas contamination and colonization may yield false-positive results on the other side [8]. The diagnosis has conventionally been left to the treating clinician based on the presence or absence of positive CSF cultures, CSF biochemical analyses, an assessment of the clinical condition, and an evaluation of the magnitude of attributable risk that infection represents for this particular patient. Thus, a recent meta-analysis found no association between VAI and increased mortality or worsened neurological outcome [3], but the author recommended the results be interpreted with caution, due to the heterogeneity of the included studies. Finally, the decision to initiate treatment implies consideration of whether continuous CSF diversion will still be needed on the short or long run.
Not surprisingly, this complex decision-making process is associated with a large variation in treatment between clinicians, hospitals, and countries [8, 38,39,40], potentially leading to both under- and overtreatment. The exploratory VAI prediction score was conducted to simplify this process, enable early prescription of adequate antibiotics in patients with VAI, and avoid or minimize inadequate treatment in those without VAI. This attempt proved largely unsuccessful, which points to the need for further, more in-depth analysis of clinical and biochemical features in large cohorts.
Strengths and limitations
The strength of this study is the large sample size, considering that the cohort consisted exclusively of patients with acute brain injury. There are, however, also several limitations, most notably the retrospective design with weaknesses regarding available and standardized data. The uneven distribution between the development and validation cohort was an unfortunate choice on our part. The quantitative information did not include basic clinical information such as the level of consciousness, body temperature, systemic antibiotic treatment, or clinical signs of VAI such as neck stiffness, which could impact the study’s reliability. Notably, the prevalence of complications such as infections may be severely mis-estimated by retrospective data sampling. The statistical analyses did not adjust for covariance in the patient samples. The criteria suggested by Willer-Hansen et al. for ruling out VAI [13] were developed for TBI patients but were tested in a more diverse cohort in the present study. This study originates from the same neurointensive care unit, and some patients featured in both studies. Finally, the study protocol was not pre-published.
Conclusion
In this study of 683 EVD-treated patients with acute brain injury, a previously suggested cut-off for ruling out VAI, consisting of a low CSF WBC/RBC ratio, a high CSF/plasma glucose ratio, and a low CSF protein, performed satisfactorily. In the multivariable logistic regression model, a longer duration from insertion to sampling and CSF WBC/RBC ratio were found independent predictors for a positive CSF culture.
Abbreviations
- AUC:
-
Area under the curve
- CSF:
-
Cerebrospinal fluid
- EVD:
-
External ventricular drain
- ICP:
-
Intracranial pressure
- IT:
-
Intrathecal
- mg:
-
Milligrams
- NG:
-
Neutrophil granulocytes
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- RBC:
-
Red blood cells
- SAH:
-
Subarachnoid hemorrhage
- Sd:
-
Standard deviation
- VA shunt:
-
Ventriculoatrial shunt
- VAI:
-
Ventriculostomy associated infections
- VP shunt:
-
Ventriculoperitoneal shunt
- VRI:
-
Ventriculostomy related infections
- TBI:
-
Traumatic brain injury
- WBC:
-
White blood cells
- 95%CI:
-
95% confidence interval
References
Lei C, De Stefano FA, Heskett C, Fry L, Le K, Brake A, Chatley K, Peterson J, Ebersole K (2023) A bibliometric analysis of the top 50 most influential articles on external ventricular drains. World Neurosurg 172:35–42. https://doi.org/10.1016/j.wneu.2023.01.040
Thamjamrassri T, Yuwapattanawong K, Chanthima P, Vavilala MS, Lele AV (2022) A narrative review of the published literature, hospital practices, and policies related to external ventricular drains in the United States: the external ventricular drain publications, practices, and policies (EVDPoP) Study. J Neurosurg Anesthesiol 34:21–28. https://doi.org/10.1097/ANA.0000000000000694
Chadwick S, Donaldson L, Janin P, Darbar A, Sutherland R, Flower O, Hammond N, Parkinson J, Delaney A (2023) The association between ventriculostomy – related infection and clinical outcomes: a systematic review and meta-analysis. J Clin Neurosci 110:80–91. https://doi.org/10.1016/j.jocn.2023.02.005
Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM (2001) Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis 33:2028–2033. https://doi.org/10.1086/324492
dos Santos SC, Fortes Lima TT, Lunardi LW, Stefani MA (2017) External ventricular drain–related infection in spontaneous intracerebral hemorrhage. World Neurosurg 99:580–583. https://doi.org/10.1016/j.wneu.2016.12.071
Dorresteijn KRIS, Jellema K, Van De Beek D, Brouwer MC (2019) Factors and measures predicting external CSF drain-associated ventriculitis: a review and meta-analysis. Neurology 93:964–972. https://doi.org/10.1212/WNL.0000000000008552
Lewis A, Wahlster S, Karinja S, Czeisler BM, Kimberly WT, Lord AS (2016) Ventriculostomy-related infections: the performance of different definitions for diagnosing infection. Br J Neurosurg 30:49–56. https://doi.org/10.3109/02688697.2015.1080222
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES (2002) Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 51:170–181; discussion 181-2. https://doi.org/10.1097/00006123-200207000-00024
Ramanan M, Shorr A, Lipman J (2021) Ventriculitis: infection or inflammation. Antibiot (Basel, Switzerland) 10. https://doi.org/10.3390/antibiotics10101246
Busl KM (2017) Nosocomial infections in the neurointensive care unit. Neurol Clin 35:785–807. https://doi.org/10.1016/j.ncl.2017.06.012
Li Y, Liu C, Xiao W, Song T, Wang S (2020) Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care 32:272–285. https://doi.org/10.1007/s12028-019-00773-w
Desai A, Lollis SS, Missios S, Radwan T, Zuaro DE, Schwarzman JD, Duhaime A-C (2009) How long should cerebrospinal fluid cultures be held to detect shunt infections? J Neurosurg Pediatr 4:184–189. https://doi.org/10.3171/2009.4.PEDS08279
Willer-Hansen RS, Olsen MH, Hauerberg J, Johansen HK, Andersen ÅB, Møller K (2022) Diagnostic criteria of CNS infection in patients with external ventricular drainage after traumatic brain injury: a pilot study. Acta Anaesthesiol Scand 66:507–515. https://doi.org/10.1111/aas.14036
Nigrovic LE, Kimia AA, Shah SS, Neuman MI (2012) Relationship between cerebrospinal fluid glucose and serum glucose. N Engl J Med 366:576–578. https://doi.org/10.1056/NEJMc1111080
Brooks M, Duong D, Shivapathasundram G, Sheridan M (2022) Cerebrospinal fluid white cell count to red cell count ratio as a predictor of ventriculitis in patients with external ventricular drains. ANZ J Surg 92:3278–3282. https://doi.org/10.1111/ans.17725
Citerio G, Signorini L, Bronco A, Vargiolu A, Rota M, Latronico N, Infezioni LIquorali Catetere Correlate Study Investigators (2015) External ventricular and lumbar drain device infections in ICU patients: a prospective multicenter Italian study. Crit Care Med 43:1630–1637. https://doi.org/10.1097/CCM.0000000000001019
Grille P, Verga F, Biestro A (2017) Diagnosis of ventriculostomy-related infection: Is cerebrospinal fluid lactate measurement a useful tool? J Clin Neurosci 45:243–247. https://doi.org/10.1016/j.jocn.2017.07.031
Hernández Ortiz OH, García García HI, Muñoz Ramírez F, Cardona Flórez JS, Gil Valencia BA, Medina Mantilla SE, Moreno Ochoa MJ, Sará Ochoa JE, Jaimes F (2018) Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg 128:262–271. https://doi.org/10.3171/2016.10.JNS16379
Lenski M, Biczok A, Neufischer K, Tonn J-C, Briegel J, Thon N (2019) Significance of cerebrospinal fluid inflammatory markers for diagnosing external ventricular drain-associated ventriculitis in patients with severe traumatic brain injury. Neurosurg Focus 47:E15. https://doi.org/10.3171/2019.8.FOCUS19407
Lenski M, Huge V, Schmutzer M, Ueberschaer M, Briegel J, Tonn J-C, Schichor C, Thon N (2019) Inflammatory markers in serum and cerebrospinal fluid for early detection of external ventricular drain-associated ventriculitis in patients with subarachnoid hemorrhage. J Neurosurg Anesthesiol 31:227–233. https://doi.org/10.1097/ANA.0000000000000496
Montes K, Jenkinson H, Habib OB, Esquenazi Y, Hasbun R (2019) Corrected white blood cell count, cell index, and validation of a clinical model for the diagnosis of health care-associated ventriculitis and meningitis in adults with intracranial hemorrhage. Clin Neurol Neurosurg 178:36–41. https://doi.org/10.1016/j.clineuro.2019.01.012
Pfisterer W, Mühlbauer M, Czech T, Reinprecht A (2003) Early diagnosis of external ventricular drainage infection: results of a prospective study. J Neurol Neurosurg Psychiatry 74:929–932. https://doi.org/10.1136/jnnp.74.7.929
Wong GK, Poon WS, Ip M (2008) Use of ventricular cerebrospinal fluid lactate measurement to diagnose cerebrospinal fluid infection in patients with intraventricular haemorrhage. J Clin Neurosci 15:654–655. https://doi.org/10.1016/j.jocn.2007.03.011
Camacho EF, Boszczowski Í, Basso M, Jeng BCP, Freire MP, Guimarães T, Teixeira MJ, Costa SF (2011) Infection rate and risk factors associated with infections related to external ventricular drain. Infection 39:47–51. https://doi.org/10.1007/s15010-010-0073-5
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJJ (2008) Risk factors for infections related to external ventricular drainage. Acta Neurochir 150:209–214. https://doi.org/10.1007/s00701-007-1458-9
Zhu Y, Wen L, You W, Wang Y, Wang H, Li G, Chen Z, Yang X (2021) Influence of ward environments on external ventricular drain infections: a retrospective risk factor analysis. Surg Infect 22:211–216. https://doi.org/10.1089/sur.2019.355
Dorresteijn KRIS, Verheul RJ, Ponjee GAE, Tewarie RN, Müller MCA, van de Beek D, Brouwer MC, Jellema K (2022) Diagnostic accuracy of clinical signs and biochemical parameters for external ventricular CSF catheter-associated infection. Neurol Clin Pract 12:298–306. https://doi.org/10.1212/CPJ.0000000000200059
Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly ES (2011) Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: a systematic review. Neurosurgery 68:996–1005. https://doi.org/10.1227/NEU.0b013e3182096d84
Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, Hamilton AJ (2003) Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg 98:725–730. https://doi.org/10.3171/jns.2003.98.4.0725
Sheppard JP, Ong V, Lagman C, Udawatta M, Duong C, Nguyen T, Prashant GN, Plurad DS, Kim DY, Yang I (2020) Systemic antimicrobial prophylaxis and antimicrobial-coated external ventricular drain catheters for preventing ventriculostomy-related infections: a meta-analysis of 5242 cases. Neurosurgery 86:19–29. https://doi.org/10.1093/neuros/nyy522
Williams TA, Leslie GD, Dobb GJ, Roberts B, van Heerden PV (2011) Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. J Neurosurg 115:1040–1046. https://doi.org/10.3171/2011.6.JNS11167
Garg K, Gupta D, Singh M, Chandra PS, Kale SS (2022) Comparison of a bolt-connected external ventricular drain with a tunneled external ventricular drain — a narrative review and meta-analysis. Neurosurg Rev 45:937–949. https://doi.org/10.1007/s10143-021-01639-6
Koopman I, Zuithoff NPA, Rinkel GJE, Vergouwen MDI (2020) The course of cerebrospinal fluid parameters ≤ 20 days after aneurysmal subarachnoid hemorrhage. J Neurol Sci 415:116899. https://doi.org/10.1016/j.jns.2020.116899
Muñoz-Gómez S, Wirkowski E, Cunha BA (2015) Post craniotomy extra-ventricular drain (EVD) associated nosocomial meningitis: CSF diagnostic criteria. Heart Lung 44:158–160. https://doi.org/10.1016/j.hrtlng.2015.01.003
Boeer K, Vogelsang H, Deufel T, Pfister W, Kiehntopf M (2011) Immediate diagnosis of ventriculits: evaluation of parameters independent of microbiological culture. Acta Neurochir 153:1797–1805. https://doi.org/10.1007/s00701-011-1079-1
Zheng G, Ji X, Yu X, Liu M, Huang J, Zhang L, Guo D, Zhang G (2020) Development and verification of a discriminate algorithm for diagnosing post-neurosurgical bacterial meningitis—a multicenter observational study. J Clin Lab Anal 34. https://doi.org/10.1002/jcla.23069
Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR (2017) 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 64:e34–e65. https://doi.org/10.1093/cid/ciw861
Bertuccio A, Marasco S, Longhitano Y, Romenskaya T, Elia A, Mezzini G, Vitali M, Zanza C, Barbanera A (2023) External ventricular drainage: a practical guide for neuro-anesthesiologists. Clin Pract 13:219–229. https://doi.org/10.3390/clinpract13010020
Siddique HH, Elkambergy H, Bayrlee A, Abulhasan YB, Roser F, Dibu JR (2022) Management of external ventricular drains and related complications: a narrative review. Curr Treat Options Neurol 24:347–363. https://doi.org/10.1007/s11940-022-00725-4
von Spreckelsen N, Jung N, Telentschak S, Hampl J, Goldbrunner R, Grau S (2018) Current treatment concepts for iatrogenic ventriculitis: a nationwide survey in Germany. Acta Neurochir 160:505–508. https://doi.org/10.1007/s00701-017-3393-8
Funding
Open access funding provided by National Hospital No funding was received for this research. The salary for Pernille Nielsen was covered by Copenhagen Neuroanaesthesiology and Neurointensive Care Research Group (CONICA).
Author information
Authors and Affiliations
Contributions
Pernille Nielsen, Markus Harboe Olsen, Rasmus Stanley Willer-Hansen, John Hauerberg, and Kirsten Møller conceived the study. The study was designed and planned by all authors. Analysis was performed by Pernille Nielsen in cooperation with Markus Harboe Olsen. All authors participated in interpretation of the data, drafting, and revising the manuscript. The final manuscript has been approved by all authors, and all authors vouch for accuracy and integrity of the manuscript content.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, no formal consent is required. The study was approved by the Directors of the Neuroscience Centre and the Departments of Neurosurgery and Neuroanaesthesiology, respectively, at Rigshospitalet (protocol id: 20069795), and approved as a register study from Centre of Regional Development (file number: R-21047752). According to Danish law, no other approval was necessary.
Conflict of interest
Jenny Dahl Knudsen is a part of Regional and national groups making guidelines for antimicrobial therapy. The remaining authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Supplemental material (DOCX 513 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nielsen, P., Olsen, M., Willer-Hansen, R. et al. Ventriculostomy-associated infection (VAI) in patients with acute brain injury—a retrospective study. Acta Neurochir 166, 128 (2024). https://doi.org/10.1007/s00701-024-06018-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06018-w
Keywords
- Acute brain injury
- Ventriculostomy associated infections
- EVD infections
- Central nervous system infections