Abstract
Purpose
Von Hippel-Lindau (VHL) is a rare inherited disease mainly characterized by the growth of tumours, predominantly hemangioblastomas (Hbs) in the CNS and retina, and renal carcinomas. The natural history of VHL disease is variable, differing in the age of onset and its penetrance, even among relatives. Unfortunately, sometimes VHL shows more severe than average: the onset starts in adolescence, and surgeries are required almost every year. In these cases, the factor that triggers the appearance and growth of Hbs usually remains unknown, although additional mutations are suspected.
Methods
We present the case of a VHL patient whose first surgery was at 13 years of age. Then, along his next 8 years, he has undergone 5 surgeries for resection of 10 CNS Hbs. To clarify this severe VHL condition, DNA from a CNS Hb and white blood cells (WBC) was sequenced using next-generation sequencing technology.
Results
Massive DNA sequencing of the WBC (germ line) revealed a pathogenic mutation in CHEK2 and the complete loss of a VHL allele (both tumour suppressors). Moreover, in the tumour sample, several mutations, in BRAF1 and PTPN11 were found. Familiar segregation studies showed that CHEK2 mutation was in the maternal lineage, while VHL was inherited by paternal lineage.
Conclusions
Finally, clinical history correlated to the different genotypes in the family, concluding that the severity of these VHL manifestations are due to both, VHL-and-CHEK2 mutations. This case report aims to notice the importance of deeper genetic analyses, in inherited rare diseases, to uncover non-expected mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Von Hippel–Lindau (VHL) disease is an autosomal dominant tumoral syndrome caused by germline mutations of the VHL tumour suppressor gene (NC_000003.12) situated at chromosome 3 (3p25-p26) [8, 10].
With an incidence of 1 in 36,000 births [7], and an almost complete penetrance (>90%) by 65 years old [8], patients may develop hemangioblastomas (Hbs) at the central nervous system (CNS).
The natural history of VHL shows that the average onset is at 33 years old and that 80% of the patients will bear, at least, 1 CNS Hb in their lifetime [22, 24]. Furthermore, visceral lesions such as, clear-cell renal cell carcinoma (ccRCC), pheochromocytomas, pancreatic neuroendocrine tumours, and benign cyst adenomas of the adnexal organ, appear in those patients. The CNS tumours include Hbs of retina and the cranio-spinal axis (cerebellum, brain stem, and spinal cord) as well as endolymphatic sac tumours (ELSTs) [7, 18]. CcRCC and its metastasis constitute the most frequent cause of mortality associated to the VHL disease, while the development and progression of retinal Hbs and CNS Hbs are cause of a high morbidity and loss of quality of life, such as blindness due to retinal detachment and the need of surgeries to control the associated symptoms [9].
Without an effective treatment for VHL tumours, repeated surgeries become the first line treatment to tackle the disease [5]. Unfortunately, continued surgeries decrease the quality of life of the patient [6]. Inhibitors of VEGF/VEGFR and mTOR have been tested in clinical trials, showing a limited success [14]. More precisely, in advanced ccRCC, tyrosine kinase inhibitors such as Pazopanib have been used, but resistance appears as adaptation to the tumour environment [26]. Recently, belzutifan, a selective HIF-2α inhibitor for VHL RC, showed a limited response in a trial on 61 VHL patients suffering from early-stage non-metastatic ccRCCs [17, 26].
Clinical diagnosis of VHL is as follows: when there is a relative affected, the presence of a single CNS Hb, pheochromocytoma or ccRCC confirms the diagnosis; while in the absence of familiar history, the presence of 2 CNS Hbs, or 1 CNS Hb and other visceral tumour, is required. Obviously, the presence of a pathogenic mutation in the VHL gene by genetic testing is a definite criterion for the diagnosis [18].
Lonser et al. [21] and Dornbos et al. [11] described the natural history of VHL disease by means of a study including 225 VHL patients, which developed more than 2500 CNS Hbs, in a mean follow-up of 6.9 years, the observation ranging from 2.1 to 9.0 years. Most tumours (72 %) grew following a saltatory pattern (periods of quiescence followed by periods of growth). In a minor proportion, tumours progressed following exponential and linear patterns (22% and 6%, respectively) [11]. Finally, only 159 (6.3%) of all Hbs became symptomatic, requiring surgery during the follow up (6.9 years).
Zhang et al. aimed to elucidate the genotype–phenotype correlations and clinical outcomes in VHL patients with large deletions (LDs). They concluded that VHL patients with deletion in exon 2 had an earlier onset age of ccRCC and pancreatic lesion, but the risk of ccRCC was lower in VHL patients with LDs and a BRK1 deletion. In addition, the group with earlier age of onset had poorer prognosis [19].
The histological origin of Hbs was unknown up to 2003 [18]. More recent investigations have shown that they may be originated from mesodermal derived hemangioblasts arrested during the embryonic development [15]. As normal embryologic hemangioblasts, Hb cells express markers of hemangioblasts. Among them, TBXT gene, called brachyury, a transcription factor within the T-box family of genes, VEGF receptor 2 (Flk-1), and stem cell leukaemia gene (SCL), which encodes a tissue-specific basic helix–loop–helix (bHLH) protein with a pivotal role in hemopoiesis and vasculogenesis [2]. The VHL tumour–derived stromal cells (hemangioblasts) can differentiate into hematopoietic and endothelial progenitors which may explain the anatomic distribution and variability of Hbs in VHL patients like the SCL axis of expression during embryogenesis [2].
Higher tumour burden has been recently associated to male sex and germline deletions. Patient with missense VHL mutations harbour less tumours than those with nonsense mutations and deletions [21]. The rate of Hb progression is significantly more rapid in symptomatic tumours and tumours with associated cysts [19, 21]. Patients younger than 20 years are significantly more likely to develop new tumours than those older than 40 years [21]. Consequently, surgical resection between 20 and 40 years could result in clinical stability since the risk of new tumour development decreases with age. Development of new tumours is also associated with the tumour burden at their presentation. A greater tumour burden may be indicator/consequence of other aggressive underlying pathological factors.
Knowledge and understanding of tumour pathophysiological features may help prognosis and improve the effectiveness of surgical and nonsurgical treatments and provide avenues for potential new therapies.
In this context, we studied the case of a VHL family with a member presenting a very early onset and a dramatic rate of Hb growth, requiring 8 surgeries in less than 10 years. This evolution of the disease contrasted with a more classical evolution in his relatives, bearing the same VHL familial mutation. To find a putative triggering factor, massive DNA sequencing in germline and in tumour cells was performed. An additional pathogenic mutation in a tumour suppressor gene was found, possibly becoming a modifying factor of the natural course of the disease.
With this work, we want to outline the importance of personalized analysis in medicine, especially in inherited rare diseases and oncology, to uncover non-expected mutations.
Materials and methods
Human samples
Blood samples and surgical surplus from members of a VHL family were processed in our lab. Informed consent from donors was obtained. The Ethical Committee of CSIC (Spanish National Research Council) approved all the procedures (references 075/2017 and 228/2020).
DNA total extraction and sequencing
Total DNA was extracted from PBL (peripheral blood lymphocytes) and fresh tumour pieces using the QIAamp Mini Kit (Qiagen, Düsseldorf, Germany) and following the manufacturer indications. In the case of buccal swabs, DNA extraction was also made with QIAamp Mini kit (Qiagen) but with longer time of lysis in the first step. The panel used for the preparation of the library has been designed using SureSelectXT technology (Agilent Technologies, CA, USA), aimed at capturing the exons of the genes of clinical interest and the flanking splicing regions (5–20 bp). Sequencing of the library was performed on a next-generation mass sequencer, NovaSeq 6000 SystemTM (Illumina, CA; USA). The tumour sample was sequenced with a reading depth of 500×. The sequences obtained were aligned against the reference genome (GRCh38/hg38) and filtered according to specific quality criteria. Subsequently, they have been analysed for the identification of genetic variants included in exonic regions or splicing regions (at least 5 bp), including missense or nonsense mutations, synonymous mutations, indels, small insertions, or deletions found at a higher allele frequency (> 30% of germline reads and > 5% of tumour sample reads). Both processes were carried out using the DRAGENTM BioIT Platform software (Illumina, version 07.021.510.3.5.7). The identified variants were filtered and narrowed down to the study genes using the bcftools view tool (Developed by Li et al. version 1.15.1) [27].
Variant annotation was performed using the freely available online platform wANNOVAR (WGLAB, PA, USA), which compiles the main databases major databases such as ClinVar (with specific information on variants associated with a known genotype) and databases of population frequency data -dbSNP, gnomAD (Genome Aggregation Database), 1000 Genome Project, or NHLBI-ESP 6500 exons.
The pathogenicity of the variants was also estimated using CADD and the addition of selected prediction systems included in the dbNSFP database (SIFT, PolyPhen2, Mutation Taster, Mutation Assessor, LRT, FATHMM, and MetaSVM) for missense mutations. For mutations identified in splicing regions (including synonymous mutations), the effect on mRNA processing has been assessed using the SpliceAI [13], Splice Site Finder and Max-Ent-Scan prediction systems, included in the SPiCE algorithm. The conservation of nucleotide position has been evaluated according to the UCSC score ranges for the PhyloP tool.
Finally, the association of the identified variants with OMIM syndromes has been evaluated (date updated to 24 April 2022).
The nomenclature and classification of the variants is based on the guidelines of the Human Genome Variation Society (HGVS) (http://varnomen.hgvs.org/) and the American College of Medical Genetics and Genomics (ACMCG).
The analysis of CNVs (copy number variations) is a screening performed with the established parameters based on a set of control samples with the DRAGEN software (version 07.021.572.3.6.3). This algorithm allows the identification of non-recurrent CNVs associated with the patient’s phenotype following the quality criteria.
Magnetic resonance imaging
MRIs were performed using a SIEMENS, Magneton Verio, 3 Teslas, 32 channel antenna, analysed by Numaris 4 Software (SIEMENS, Munich, Germany).
For acquisition of brain MRI images from the patients, a specific VHL protocol was used, including axial T1, axial T2, axial FLAIR, T2* (gradient echo), DW (diffusion-weighted), axial T1 SPIR (fat suppression, contrast-enhanced), and 3D T1 SPACE (iso, contrast-enhanced). For the posterior fossa study (endolymphatic sac tumours), thin sections of axial T1, axial FLAIR, and 3D T2 SPACE were obtained.
For acquisition of complete spinal cord MRI, a specific VHL protocol was used, including sagittal T1, sagittal T2 Dixon, axial T2, and sagittal and axial T1 SPIR (contrast-enhanced).
Results
Genetic analysis of VHL gene in a family
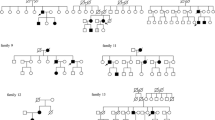
The VHL main case of study is a 23-year-old male, third generation with VHL disease and genetic diagnosis at 11 years of age. The family mutation consisted of a complete deletion of one VHL allele, being the patient hemizygous for the VHL gene in the germ line. The loss occurred on chromosome 3 at the 3p25.3 cytoband, in the genomic region chr3:10141778-10149995 (GRCh38). This mutation was inherited through the parental lineage and was also present in his sister. The VHL disease segregation among the members of the family is shown in Fig. 1A (pedigree).
The patient III-1, case of this study (Fig. 1A, black arrow) bears a complete gene deletion. In Fig. 1B, the interactive tool Integrative Genomics Viewer, (IGV) for the visual exploration of genomic data was used. He was primarily attended at other centre and then referred to our VHL unit at FJD, for follow-up and treatment of CNS tumours derived from VHL disease.
Clinical manifestations of the VHL affected members
Table 1 shows the different surgeries undergone by the VHL-affected members of the family, including: age at the VHL disease onset, date of each surgery, and type of tumours resected from the father of the patient (II-1); the patient, main case of study (III-1); and his sister (III-2). In Fig. 1C, a timeline scheme of the surgeries is shown for better comparison and easier understanding of the clinical courses of these 3 patients.
Figure 2 shows MRI images of different tumours present in the main case of study prior to surgeries.
Patient I-1
The index case of VHL disease. He was clinically diagnosed late in age, and no clinical data from this patient are available.
Patient II-1
A 55-year-old male VHL patient with onset of the disease at 42 years of age, genetically diagnosed with a complete allelic loss of VHL. This patient underwent his first surgery in other centre with removal of 2 hemispheric cerebellar Hbs in 2009, requiring a ventriculo-peritoneal shunt due to hydrocephalus. In 2010, he underwent a second surgery at FJD for removal of two Hbs located on the posterior medulla oblongata and upper cervical spinal cord. Ending 2010, additional surgery took place for removal of two other Hbs at the brain stem (nested in the obex and floor of IV ventricle), and two cerebellar Hbs (paravermal and right hemisphere). In 2014, a new surgical intervention was performed with removal of three tumour nodules, the largest in posterior medulla location and two smaller ones in a left medullary location. Then, under the same anaesthetic procedure, a second surgical intervention was performed with a left retro-sigmoid approach, for removal of a fourth tumour in the cerebellar hemisphere. Postoperative period followed without neurological deficits. Since 2014 until now, no further neurosurgeries have been needed.
Patient III-2
A 21-year-old woman, genetically diagnosed with VHL disease at the age of 9, carrier of a pathogenic mutation inherited from her father (patient II-1), who was referred in 2014 for MRI evaluation at the VHL unit of the FJD at the age of 12, being asymptomatic at that moment. In 2017, MRI showed 2 Hbs in obex (medulla oblongata) and right para-medullary region, both of 5 mm, without associated cyst. A full spine MRI showed absence of Hbs in spinal cord. Conservative management was decided with programmed MRI follow-up after 1 year. In 2018, the patient started with episodes of hiccups, coughing, and choking, presenting enlargement of the lesion located in obex (8 mm of size), while maintaining unchanged the right 5mm HGB in para-medullary region. Surgical intervention was decided to avoid the progression of symptoms and a permanent neurological deficit. The patient underwent surgery, achieving the removal of the two medullary tumours, without complications. No further neurological surgeries have been necessary since then.
Patient III-1
Son of patient II-1 and genetically diagnosed of VHL at 11 years old, asymptomatic and without tumours at the time of diagnosis. During his follow-up in 2012, an MRI showed for the first time the presence of a small left lateral medullary nodule, as well as a 7.5mm nodule in the posterior medulla oblongata. The latter grew quickly reaching 11mm in a year. Given the rapid growth and location of the lesion, he underwent a first surgery in 2013 to resect 2 medullary and 1 cervico-medullar Hbs. After surgery, the patient presented some transitory sequelae with slight loss of finger sensitivity at the right hand, and slight dysmetria in the left upper limb with complete recovery in consecutive revisions. Three months after the intervention, the patient presented a surgical wound infection and post-laminoplasty cervical kyphosis, requiring reoperation for cleaning, with anterior cervical arthrodesis C4–C5 and posterior instrumentation.
The patient remained stable for 18 months. Then, in 2015, a brain and spine MRI exam revealed the presence of a subcentimetric Hb on the right lateral medulla, without associated cyst; two more lesions were detected at C3–C4 and C6, without associated cysts; as well as a nodular Hb at the posterior conus medullaris, with associated edema and thickening of the cone. In August 2016, while the cervical and cone lesions remained stable, the lesion in the right margin of the medulla showed a millimetric growth. The lack of significant symptoms plus the millimetric growth, led to a conservative management for the next 2 years of follow-up. However, in 2018, an MRI of the whole spinal cord showed an increase of the conus medullaris lesion, with significant growth of the cyst and edema at that level and the surrounding spinal cord respectively (Fig. 3). These findings conditioned a new surgery with a complete resection of the tumour and an adequate post-surgical evolution. Nevertheless, 6 months later, in 2019, the patient presented paraesthesia on the left upper limb with radiological stability and consequently a conservative management. In January 2020, the patient presented a worsening of the neurological symptoms in the extremities. Imaging tests showed an increase in the size of the medullary lesion, as well as oedema surrounding the associated cyst. Likewise, an increase in the size of the cyst over the C4–C5 lesion was apparent, with medullary bulging and perilesional edema. Given these symptoms, in March 2020, surgery was performed on the medullary and cervical lesions, resecting a total of 3 Hbs (1 medullary and 2 cervical), with no complications. Postoperatively, the patient presented alterations in proprioceptive sensitivity and coordination in the right upper limb, which improved during hospitalization.
The follow up of the patient showed progressive improvement of the sensitive deficit in the right hand, but a persistent decrease in manual ability. The brain lesions remained stable, but significant growth of the cystic area associated with the C5 anterior Hb was apparent in MRI of the cervical spine. Conservative management was decided on this occasion. However, the evolution was to continuous cervicalgia with pain radiating to both trapezia. Given the growth of the cervical lesion cyst, the patient’s symptoms, and the surgical accessibility by posterior approach, a new intervention was decided. In October 2021, surgery was performed, and the lesion was resected without associated complications. In October 2022, the patient presented a rapid clinical worsening with uncontrollable pain at the level of the left costal grid. An MRI of the dorsal spine was performed showing significant growth of a previously known dorsal intramedullary Hb, with a large cystic cavity and associated myelopathy that had remained stable until that moment (Fig. 2). Given the radiological and clinical myelopathy, a new surgery was practiced in December 2022, achieving the removal of the lesion at D3 level, without complications and with adequate post-surgical evolution.
In the last MRI evaluation, in February 2023, a growth of the right cerebellar lesion was evident, with probable future surgical management, given the progressive growth of the lesion and the patient’s coordination alterations.
Genetic analysis from tumoral and blood DNA by NGS
Looking at the clinical symptoms of this family, (Table 1), especially at the onset age and the number of surgeries/tumours, the case of patient III-1 is striking by the early and severe VHL presentation.
In certain cases of VHL disease, the fast growth of Hbs may occur due to the accumulation of the already present loss of function (LOF) of the VHL gene with somatic mutation(s) in other tumour-related gene(s). To investigate the possibility of additional mutations in these genes, a massive sequencing analysis of total DNA from a piece of Hb coming from the last surgery (Table 1) in cerebellum of patient III.1, was performed. The massive sequencing, with a high level of readings, yielded three different mutated genes present at this tumour, in addition to the already known mutation in the inherited VHL gene.
The variant c.1259+1G>C (NM_007194.4, rs121908707) was detected in heterozygosis in the CHEK2 gene, affecting the first nucleotide of intron 10, interfering with the splice site leading to skipping of exon 10, loss of the reading frame and a premature termination of the protein sequence (p.I336Pfs*2). In silico CADD (combined annotation dependent depletion) indicated a high predictor value of 34, pointing to high probability of pathogenicity.
Analysis of the DNA from peripheral blood indicated the presence of the same mutation in the germ line, as shown in Fig. 4B (III.1).
To determine whether the mutation was inherited from the parents or was a de novo mutation in patient III.1, segregation analysis of CHEK2 mutation in the family was studied. Figure 4A shows the familiar pedigree and the Sanger sequencing of CHEK2 DNA from saliva in both parents and his sister. As seen in the DNA sequencing chromatogram, CHEK2 mutation was found in the mother but absent in the father and the sister, both VHL patients. Of note, his mother had been diagnosed of an in situ breast carcinoma, resected 5 years ago, and his maternal grandfather was diagnosed of prostate cancer. In both cases the presence of tumours correlated to the finding of the CHEK2 mutation.
In addition to CHEK2 mutation found in the tumour and inherited through the germ line, 2 more somatic mutations were detected only in the tumour sample.
The analysis of CNVs in the tumour sample showed the presence of a deletion in exon 1 of BRAF. The loss occurs on chromosome 7, at cytoband 7q34, in the genomic region chr7:140924459-140924753 (GRCh38). The BRAF proto-oncogene encodes a protein of the RAF family, composed of serine/threonine kinases that mediate cellular responses to grow signals by activating the mitogen-activated protein kinase pathway (MAPK). Additionally, the analysis also showed the presence of a deletion in exon 1 of PTPN11, a protein member of the protein tyrosine phosphatase (PTP) family. The loss occurs on chromosome 12, at cytoband 12q24.13, in the genomic region chr12:112419022-112419200 (GRCh38). The potential consequences of these mutations will be explained in the discussion section.
Discussion
Rare diseases are characterized by the low number of patients suffering from each individual disease, but also by the scarce knowledge at clinical and research level, due to their rarity. In addition to their condition, it must be considered that these patients are not free from other diseases or mutations that affect general population. Particularly, in the case of VHL disease, we have published the case of a 76-year-old woman in which a mutation in CLN5 (ceroid lipofuscinosis, neuronal, 5) offers a protective effect, preventing VHL-related tumour development [4]. Here, we introduce the other side of the coin, a mutation that could dramatically affect Hb CNS tumours raising and development.
In RDs, there are two main goals: an early diagnosis and the natural history knowledge of each disease, which contribute to a better clinical management and to find therapies improving quality of life. Knowledge of the natural history of the disease is a key point for its clinical management. For this purpose, the medical record documents from patients and review studies from them, would allow to know the expected evolution of a rare pathology.
In the case of VHL disease, makes mandatory a follow up of patients by experienced clinicians.
As mentioned in the Introduction section, surgery arises as the only way to resolve the symptoms of VHL disease. Since patients have multiple asymptomatic/symptomatic tumours in the context of the disease, surgeons must follow a conservative approach, and only remove those leading to life-threatening symptoms, deciding the proper timing in each case.
To describe and publish the natural history of CNS Hb development in VHL patients is extremely useful. Lonser et al. indicated that in 70–80% of cases, the common pattern is an evolution of growth periods followed by quiescence phases in tumour development [21].
Several clinical trials have been carried out with drugs, most of them used in cancer chemotherapy to stop and space the need of surgeries, extending the periods of tumour quiescence (angiogenesis inhibitors, HIF-2 dimerization inhibition, β-blockers, etc.). However, no clearly positive results are available so far [14].
The present work describes the particularly severe case of a VHL patient, with a very early disease onset, at the age of 11 years, and with the need of a first surgery at the age of 13 years old. Since then, a conservative surgical basis has been applied, removing only those tumours with severe clinical impact on the patient. A total of 8 neurosurgeries have been necessary, and he is currently 23 years old. The patient inherited paternally the complete deletion of a VHL allele, but both, father and grandfather had the disease with a much later onset, presenting their first symptoms after the age of 40. Regarding the father, his first surgery of the CNS was at the age of 42 and, as shown in Table 1 and Fig. 1, in a period of 4 years he underwent several surgeries. However, notably since 2014, he has remained without the need for new interventions.
It could be hypothesized that in patient III-1 (Fig. 1), there is a phenomenon of gene anticipation as published in VHL [25]. However, the present work has another explanation. In fact, the present work is a paradigmatic case of how useful a personalized medicine approach is. By massive genetic sequencing analysis of DNAs from both, tumour, and germ line (peripheral blood), we have discovered the presence of two germline mutations in the patient. He inherited an allelic loss of VHL from the paternal side and a pathogenic mutation in the tumour suppressor gene CHEK2 from the maternal side.
CHEK2 encodes the Chek2 protein kinase activated in response to DNA damage, being involved in cell cycle arrest. Mutations in CHEK2 are responsible for an increased predisposition to breast, prostate, colon, stomach, and brain cancer [20]. In relation to VHL, it has been shown that CHEK2 binds to the β-domain of pVHL and phosphorylates it upon DNA damage. Therefore, this modification enhances pVHL-mediated transactivation of p53, recruiting p300 and Tip60 to the chromatin of p53 target gene [23]. Moreover, CHEK2 functions as a DNA damage checkpoint kinase by phosphorylating p53 [16]. Bell et al. [20] described heterozygous germline mutations in the CHEK2 gene in patients with Li-Fraumeni syndrome, suggesting that CHEK2 is a tumour suppressor gene whose loss of function confers predisposition to develop sarcoma, breast cancer, and brain tumours. The CHEK2 variant found in our case had been previously identified and described in patients with non-Hodgking’s lymphoma by Havranek et al. [1].
Upon finding the CHEK2 mutation, it was hypothesized that this mutation would not be present in the rest of the family siblings carrying the VHL mutation. Thus, the segregation of CHEK2 in the family germline was carried out. As expected, it was observed that the mutation came from the maternal line (pedigree II-2). It is worth mentioning that the mother underwent a breast cancer surgery several years ago. In the family, the only case where pathogenic mutations in both genes, VHL and CHEK2, are concomitant was in patient III-1, showing an unusual and very severe clinical history of VHL disease. We, therefore, conclude that the mutation in CHEK2 is the factor triggering the rapid growth of CNS Hbs in this patient. Furthermore, at the time or writing this manuscript, Zhang et al. found two VHL patients with LDs also carrying CHEK2 and FLCN germline mutations, respectively [19].
Moreover, in other pathologies such as familial cavernomatosis, it has recently been described that somatic mutations in PIK3CA trigger the growth and bleeding of cavernomas that lead to surgeries [3]. Considering the evolution of the patient and the differences with respect to first-degree relatives (bearing a VHL mutation but lacking the CHECK2 mutation), is very likely that the combination of mutations in two different tumour suppressor genes is responsible for the more aggressive behaviour of the disease in patient III-1. Following the clinical-genetic finding, the family has been referred to familiar oncology, to study the possible benefit derived from CHEK2 mutation–targeted therapy, which could stop the continuous triggering of tumour growth.
In addition, a complete genetic analysis of the tumour revealed not only the presence of CHEK2 and VHL mutations, but also the presence of deletions in exon 1 of BRAF-1 and PTEN11, genes involved in tumour development by LOF. It is worthy to remark that these mutations were not present in the germline.
In the case of BRAF-1, a key intermediate in the RAS pathway and in the transmission of signals that regulate cell proliferation, differentiation, and survival, exon 1 corresponds to the N-terminal regulatory domain that precedes the Ras-binding domain of BRAF. In the work of Martínez-Fiesco et al. [12], they suggest that this region may represent an additional level of regulation in terms of RAS-BRAF interaction. Moreover, Terrell et al. [23] reported a high affinity of BRAF for KRAS; however, they observed that BRAF proteins lacking the N-terminal domain had an increased affinity for other RAS family proteins different from KRAS, such as HRAS and NRAS.
PTPN11, as PTP protein, regulates a variety of cellular processes, including cell growth, differentiation, the mitotic cycle, and oncogenic transformation. Mutations in PTPN11 lead to the activation of the RAS-MAPK pathway. Exon 1 is included within the SH2 N-terminal domain involved in the switching of the protein between its inactive and active conformations. Therefore, alterations in this domain can cause a significant shift in the balance, favouring the active conformation and, therefore its malignancy.
As mentioned above, another interaction in the germline was described by our group recently [4]. Fortunately, the opposite effect to the present case was produced since the tumour development due to a VHL mutation was counteracted by a germ line mutation in heterozygous condition at the CLN5 gene [4]. These findings of gene interactions between VHL and other genes in the germline underlines the need to study the whole exome in those cases in which the disease does not follow a natural course. Therefore, completing the two main points above indicated (diagnosis and natural history of the disease), massive germline and tumour NGS study should be done with all VHL patients, starting from their first surgery.
Advances in genetics and molecular biology techniques make it advisable to apply personalized medicine to monitor and treat each patient according to their genetics and associated symptoms.
Thus, by showing this case, we propose deeper genetic studies, searching for the origin of tumoral abnormal growth outside the natural history. In many cases, if we were able to find the cause, maybe the quiescence of the disease could be reached, by treating patients according to the affected gene. The study of genetic factors that can trigger the development of tumours through massive sequencing can help to modify the natural evolution of genetic tumoral diseases like VHL, in a personalized way. On the other hand, we cannot rule out that there may be also environmental or epigenetic causes triggering Hbs’ growth, and that should also be considered.
Conclusions
The present work shows the importance of personalized medicine in, the case of a VHL patient bearing a second inherited mutation, which we propose as the trigger for the growth of his CNS hemangioblastomas. VHL is a tumour suppressor gene, and we postulate that the cause of the abnormal severity in the course of his disease, is an additional mutation in CHEK2, another tumour suppressor gene. Consequently, the onset of the disease is sped up to 11 years old and, along the following 12 years, he underwent 5 surgeries (10 hemangioblastomas), while his VHL mutation-bearing relatives (father and sister) had a much later onset and underwent 4 and 1 surgeries, respectively, in their life.
Thus, our objective is to provide an explanation why some VHL patients have an accelerated growth in their hemangioblastomas and to highlight the importance of exhaustive genetic analyses to uncover mutations in additional genes that can modulate the development of the disease and establish therapeutic targets.
Code Availability
Not applicable.
Data Availability
Not applicable.
Abbreviations
- ACMCG:
-
American College of Medical Genetics and Genomics
- bHLH:
-
Basic helix–loop–helix
- CADD:
-
Combined annotation dependent depletion
- ccRCC:
-
Clear cell renal cell carcinoma
- CLN5:
-
Ceroid lipofuscinosis neuronal
- CNS:
-
Central nervous system
- CNVs:
-
Copy number variations
- DW:
-
Diffusion-weighted
- ELST:
-
Endolymphatic sac tumour
- Hbs :
-
Hemangioblastomas
- HGVS :
-
Human Genome Variation Society
- HIF:
-
Hypoxia inducible factor
- IGV:
-
Integrative Genomics Viewer
- LOF:
-
Loss of function
- MRI:
-
Magnetic resonance imaging
- NGS:
-
Next generation sequencing
- PBL:
-
Peripheral blood lymphocytes
- PTP:
-
Protein tyrosine phosphatase
- RBD:
-
Ras-binding domain
- RC:
-
Renal carcinoma
- RD:
-
Rare disease
- SCL:
-
Stem cell leukaemia
- SNPs:
-
Single nucleotide polymorphisms
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- VHL:
-
von Hippel-Lindau
- WBC:
-
White blood cells
References
Albiñana V, Gallardo-Vara E, de Rojas PI, Recio-Poveda L, Aguado T, Canto-Cano A, Aguirre DT, Serra MM, Gonzalez-Peramato P, Martinez-Pineiro L, Cuesta AM, Botella LM (2020) Targeting beta2-adrenergic receptors shows therapeutical benefits in clear cell renal cell carcinoma from Von Hippel-Lindau disease. J Clin Med 9. https://doi.org/10.3390/jcm9092740
Aronoff L, Malkin D, Kv E, Gallinger B, Wasserman J, Kim RH, Villani A, Meyn MS, Druker H (2018) Evidence for genetic anticipation in vonHippel-Lindau syndrome. J Med Genet 55:395–402. https://doi.org/10.1136/jmedgenet-2017-104882
Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA (1999) Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286:2528–2531. https://doi.org/10.1126/science.286.5449.2528
Binderup ML, Jensen AM, Budtz-Jorgensen E, Bisgaard ML (2017) Survival and causes of death in patients with von Hippel-Lindau disease. J Med Genet 54:11–18. https://doi.org/10.1136/jmedgenet-2016-104058
Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, Michaelson MD, Appleman LJ, Thamake S, Perini RF, Zojwalla NJ, Jonasch E (2021) Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 27:802–805. https://doi.org/10.1038/s41591-021-01324-7
Cybulski C, Gorski B, Huzarski T, Masojc B, Mierzejewski M, Debniak T, Teodorczyk U, Byrski T, Gronwald J, Matyjasik J, Zlowocka E, Lenner M, Grabowska E, Nej K, Castaneda J, Medrek K, Szymanska A, Szymanska J, Kurzawski G et al (2004) CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet 75:1131–1135. https://doi.org/10.1086/426403
de Rojas PI, Albiñana V, Recio-Poveda L, Rodriguez-Rufian A, Cuesta AM, Botella LM (2020) CLN5 in heterozygosis may protect against the development of tumors in a VHL patient. Orphanet J Rare Dis 15:132. https://doi.org/10.1186/s13023-020-01410-y
Dornbos D 3rd, Kim HJ, Butman JA, Lonser RR (2018) Review of the neurological implications of von Hippel-Lindau disease. JAMA Neurol 75:620–627. https://doi.org/10.1001/jamaneurol.2017.4469
Feletti A, Anglani M, Scarpa B, Schiavi F, Boaretto F, Zovato S, Taschin E, Gardi M, Zanoletti E, Piermarocchi S, Murgia A, Pavesi G, Opocher G (2016) Von Hippel-Lindau disease: an evaluation of natural history and functional disability. Neuro-Oncology 18:1011–1020. https://doi.org/10.1093/neuonc/nov313
Havranek O, Kleiblova P, Hojny J, Lhota F, Soucek P, Trneny M, Kleibl Z (2015) Association of germline CHEK2 gene variants with risk and prognosis of non-Hodgkin lymphoma. PLoS One 10:e0140819. https://doi.org/10.1371/journal.pone.0140819
Heidegger I, Pircher A, Pichler R (2019) Targeting the tumor microenvironment in renal cell cancer biology and therapy. Front Oncol 9:490. https://doi.org/10.3389/fonc.2019.00490
Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, Kosmicki JA, Arbelaez J, Cui W, Schwartz GB, Chow ED, Kanterakis E, Gao H, Kia A, Batzoglou S, Sanders SJ, Farh KK-H (2019) Predicting splicing from primary sequence with deep learning. Cell 176:535–548.e524. https://doi.org/10.1016/j.cell.2018.12.015
Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan V, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, Thamake S, Park EK, Zojwalla NJ, Perini RF, Linehan WM, Srinivasan R (2020) Phase II study of the oral HIF-2α inhibitor MK-6482 for Von Hippel-Lindau disease–associated renal cell carcinoma. J Clin Oncol 38:5003–5003. https://doi.org/10.1200/JCO.2020.38.15_suppl.5003
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L et al (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260:1317–1320. https://doi.org/10.1126/science.8493574
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH (2003) von Hippel-Lindau disease. Lancet 361:2059–2067. https://doi.org/10.1016/S0140-6736(03)13643-4
Lonser RR, Butman JA, Huntoon K, Asthagiri AR, Wu T, Bakhtian KD, Chew EY, Zhuang Z, Linehan WM, Oldfield EH (2014) Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J Neurosurg 120:1055–1062. https://doi.org/10.3171/2014.1.JNS131431
Lonser RR, Butman JA, Kiringoda R, Song D, Oldfield EH (2009) Pituitary stalk hemangioblastomas in von Hippel-Lindau disease. J Neurosurg 110:350–353. https://doi.org/10.3171/2008.4.17532
Martinez Fiesco JA, Durrant DE, Morrison DK, Zhang P (2022) Structural insights into the BRAF monomer-to-dimer transition mediated by RAS binding. Nat Commun 13:486. https://doi.org/10.1038/s41467-022-28084-3
Neumann HP, Wiestler OD (1991) Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet 337:1052–1054. https://doi.org/10.1016/0140-6736(91)91705-y
Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, Sohn T, Kim SH, Lubensky IA, Vortmeyer AO, Rodgers GP, Oldfield EH, Lonser RR (2007) von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 4:e60. https://doi.org/10.1371/journal.pmed.0040060
Ren AA, Snellings DA, Su YS, Hong CC, Castro M, Tang AT, Detter MR, Hobson N, Girard R, Romanos S, Lightle R, Moore T, Shenkar R, Benavides C, Beaman MM, Muller-Fielitz H, Chen M, Mericko P, Yang J et al (2021) PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature 594:271–276. https://doi.org/10.1038/s41586-021-03562-8
Terrell EM, Durrant DE, Ritt DA, Sealover NE, Sheffels E, Spencer-Smith R, Esposito D, Zhou Y, Hancock JF, Kortum RL, Morrison DK (2019) Distinct binding preferences between Ras and Raf family members and the impact on oncogenic Ras signaling. Mol Cell 76(872-884):e875. https://doi.org/10.1016/j.molcel.2019.09.004
Van Velthoven V, Reinacher PC, Klisch J, Neumann HP, Glasker S (2003) Treatment of intramedullary hemangioblastomas, with special attention to von Hippel-Lindau disease. Neurosurgery 53:1306–1313; discussion 1313-1304. https://doi.org/10.1227/01.neu.0000093497.81390.29
Wait SD, Vortmeyer AO, Lonser RR, Chang DT, Finn MA, Bhowmick DA, Pack SD, Oldfield EH, Zhuang Z (2004) Somatic mutations in VHL germline deletion kindred correlate with mild phenotype. Ann Neurol 55:236–240. https://doi.org/10.1002/ana.10807
Wanebo JE, Lonser RR, Glenn GM, Oldfield EH (2003) The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg 98:82–94. https://doi.org/10.3171/jns.2003.98.1.0082
Zhang K, Yang W, Ma K et al (2023) Genotype-phenotype correlations and clinical outcomes of patients with von Hippel-Lindau disease with large deletions. J Med Genet 60(5):477–483. https://doi.org/10.1136/jmg-2022-108633
Acknowledgements
The authors want to thank in the first place the Spanish Alliance of VHL, for direct support, inspiration, and help from the patients who donated surgical surplus for our research. Authors recognize specially the help of the family involved in this manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Ministry of Science and Innovation of Spain (grant number PID2020-115371RB-I00).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.C.M., D.T.A, A.M.C, and L-M.B; methodology, J.C.M., D.T.A., L.L.H., and L.R.P.; software, J.V.L. and J.P.P.; validation, J.V.L., J.P.P., V.A., L.R.P., L.M.B., and A.M.C.; formal analysis, J.V.L., V.A., A.M.C., and L.M.B.; investigation, L.L.H., L.R.P., V.A., L.M.B., and A.M.C.; surgical surplus and clinical data, J.C.M., D.T.A.; writing-original draft preparation, A.M.C and L.M.B.; writing-review and editing, J.C.M., D.T.A, J.V.L., J.P.P., L.L.H., L.R.P., V.A., L.M.B., and A.M.C.; funding acquisition, L.M.B.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Spanish National Research Council (CSIC) (references 075/2017 and 228/2020) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cabrera-Montes, J., Aguirre, D.T., Viñas-López, J. et al. Mutation in Chek2 triggers von Hippel-Lindau hemangioblastoma growth. Acta Neurochir 165, 4241–4251 (2023). https://doi.org/10.1007/s00701-023-05825-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05825-x