Abstract
Objective
To refine a reliable and reproducible intraoperative visual evoked potentials (iVEPs) monitoring protocol during endoscopic transsphenoidal surgery. To assess the reliability of baseline iVEPs in predicting preoperative visual status and perioperative iVEP variation in predicting postoperative visual outcome.
Methods
Sixty-four patients harboring tumors of the pituitary region were included. All patients underwent endoscopic endonasal approach (EEA) with iVEPs monitoring, using a totally intravenous anesthetic protocol. Ophthalmological evaluation included visual acuity and visual field studies.
Results
Preoperatively, visual acuity was reduced in 86% and visual field in 76.5% of cases. Baseline iVEPs amplitude was significantly correlated with preoperative visual acuity and visual field (p = 0.001 and p = 0.0004, respectively), confirming the reliability of the neurophysiological/anesthetic protocol implemented.
Importantly, perioperatively the variation in iVEPs amplitude was significantly correlated with the changes in visual acuity (p < 0.0001) and visual field (p = 0.0013). ROC analysis confirmed that iVEPs are an accurate predictor of perioperiative visual acuity improvement, with a 100% positive predictive value in patients with preoperative vision loss.
Conclusions
iVEPs during EEA is highly reliable in describing preoperative visual function and can accurately predict postoperative vision improvement.
Significance
iVEPs represent a promising resource for carrying out a more effective and safe endoscopic transsphenoidal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visual deterioration is the most common neurological sign of an enlarging mass in the pituitary region: during tumor removal, monitoring visual function to detect promptly worsening or amelioration would help the surgeon in deciding the most appropriate conduction.
Visual disturbances are caused by tumor compression on the different portions of the optic pathways but may also be the consequence of surgical tumor removal. In fact, a direct damage may follow the dissection of a stiff tumor from the optic structures while an indirect injury may occur by coagulating its vascular supply. The endonasal endoscopic approach (EEA) and its extended variant are the gold standard for the surgical treatment of pathologies of the hypothalamic-pituitary region: enhanced visualization and magnification of the anatomical structures facilitate a more complete tumor removal. Then, a more accurate neurophysiological monitoring is warranted for a safe surgery [1, 6, 32].
To achieve this goal, the monitoring of intraoperative visual evoked potentials (iVEPs) is a possible resource: in the last few years, there have been reports on its clinical application, but more data are necessary to assess reliability and predictive value. In fact, differently from SSEP and MEP, VEPs present a high intra-individual variability and relative instability/susceptibility of the acquisitions, limiting their wide clinical use [4, 8, 14, 15, 17, 19, 22, 28].
On this basis, a novel VEP acquisition protocol was established and reproduced for all patients included in the study, performing TIVA anesthesia, using a short duration, high intensity white light LED flash stimulus to optimize visual response evocation, and monitoring with electroretinogram and electroencephalogram, respectively, the visual stimulus and the correct neuroanaesthesiological level. As far as is known, not similar VEP protocols are reported in the literature.
For these reasons, the aims of the present study are (1) to develop a reliable and reproducible protocol for iVEPs in EEA; (2) to verify the reliability of baseline iVEPs in describing the preoperative visual status; and (3) to verify the reliability of the iVEPs in predicting the postoperative visual outcome.
Methods
A prospective study was conducted to evaluate the feasibility and reliability of iVEPs monitoring on patients undergoing EEA for the treatment of sellar-suprasellar lesions, in a single tertiary institution. This study was approved by the Institutional Ethics Committee (Prot. 17814/19, ID: 2557). Enrolled patients signed an informed consent form; consent for publication of protected health information was obtained.
Enrollment and inclusion criteria
We included in the study all consecutive patients operated upon for sellar-suprasellar lesions with clinical and radiological evidence of optic pathways impairment in the period October 2019–July 2021. Patients < 18 years of age and patients with primary or secondary blindness were excluded.
Baseline preoperative evaluation
All patients underwent to preoperative ophthalmological evaluation (visual acuity with Snellen chart and visual field test with computerized perimetry).
Intraoperative visual evoked potentials (iVEPs)
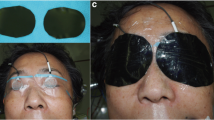
iVEPs monitoring was carried out with white light LED flash stimulation delivered by 3 LEDs with a power of 5500 lx each (total power 16,500 lx). The hardware used was the Medtronic NIM-ECLIPSE® E4 and the stimulation was performed with Inomed LED flash goggles in a mono and binocular manner, fixed on the closed eyelids with adhesive plastic and plaster (Fig. 1). iVEPs were acquired at regular intervals as described in this paragraph. The duration of the stimulus was 1 ms and the stimulation frequency 1.1 Hz. The recording was performed by using corkscrew electrodes, positioned in O1, O2, and Oz position according to the 10–20 international system with a biauricular reference (A1 + A2). The acquisition time was 300 ms with an average of at least 100 traces to reduce the artefactual component. iVEPs component studied is the N2-P2 [20]. N2-P2 is the second negative and positive peak, respectively. The latency of this wave is near 100 ms and is considered as the P100 of the pattern visual evoked potentials. Bilateral ERG was performed to be sure of retinal activation and to avoid false positive responses since a consensual reduction or loss of ERG and iVEPs suggests a technical problem [16]. Stimulation parameters of ERG were the same of iVEPs while recording electrodes were positioned on the ipsilateral, lateral cantus oculi (referred to nasion).
iVEPs recording was performed at different steps as follows:
-
1)
Baseline iVEPs: acquired under anesthesia, after disinfection and surgical dressing, before entering the endoscope into the nostrils.
-
2)
Perioperative iVEPs: during the surgical procedure, from the opening of the sellar dura iVEPs were acquired regularly at 5′ intervals, marking down the most significant steps as “dura opening,” “tumor debulking,” “tumor capsule dissection,” “sellar reconstruction,” and any other surgical maneuver regarded as relevant.
-
3)
Final iVEPs: acquired at the end of surgery with the patient still under anesthesia, before removing the surgical dressing.
Variations in the iVEP amplitude > 10% compared to baseline were considered significant. The changes in the iVEP amplitude occured during surgery and remained until the last acquisition made at the end of the procedure were defined as “stable.”
iVEP latency variations were also considered in the preliminary analysis. However, its changes were minimal, were not statistically significant and therefore were not further considered.
Neuro-anesthetic protocol
The anesthetic technique used was TIVA (Total IntraVenous Anesthesia) with propofol and remifentanil both TCI (Target Controlled Infusion) [25, 29, 30]. Details are provided in Supplementary Methods. Bispectral index (BIS) was kept stable at values 45–50 throughout the VEP recording phase.
Endoscopic endonasal approach (EEA)
Patients were operated on by a multidisciplinary team consisting of Otolaryngologist and Neurosurgeon with extensive experience in EEA. The technique used was the binostril transsphenoidal approach with posterior septostomy, as previously described [5, 12]. Details are provided in Supplementary Methods.
Postoperative evaluation and follow-up
The first postoperative evaluation was performed 72 h after surgery: visual acuity and visual field testing were performed with the same modalities of baseline. The tests were then repeated at 3 months follow-up, establishing the final result of visual acuity and visual field for each patient, used for the statistical analysis.
Improved or worsened changes of at least 1/10 in visual acuity were considered significant. For visual field, variations of at least one campimetric quadrant were considered significant.
Statistical analysis
We analyzed separately visual acuity, visual field, and iVEPs of each eye (n = 128). Continuous values were described using mean ± standard deviation (SD), categorical variables using absolute and relative frequencies. Correlation between continuous variables was performed by plotting regression lines and calculating the Spearman correlation coefficient. Comparison of continuous variables between groups was performed using the Mann–Whitney U test, and comparison of categorical variables was performed using the chi-square statistics adopting the Fisher exact test when appropriate. To assess the reliability of perioperative VEP variation in predicting visual change, we plotted ROC curves, calculated the area under the curve (AUC) and assessed the best cutoff. The latter was defined as the threshold value at which the Youden index (i.e., the difference between sensitivity and 1-specificity), has its maximum value. Analyses were performed using StatView ver 5.0 (SAS Institute, Cary, NC, USA) and MedCalc ver 20.015 (MedCalc Software Ltd, Osted, Belgium).
Results
Study population
Sixty-four patients were included (Table 1). The mean age at diagnosis was 57.7 years (range 18–84); the male/female ratio was 31/33. The most frequent histological diagnoses were pituitary adenoma (29 cases, 45.3%), meningioma (12 cases, 18.7%), and craniopharyngioma (10 cases, 15.6%) (Fig. 2).
The preoperative visual function assessment revealed a reduction in vision in at least one of the two eyes in 55 patients (85.9%) and a reduction in the visual field in at least one of the two eyes in 49 patients (76.5%).
Postoperative visual outcome
Out of 128 eyes of the study, visual acuity improved in 54 (42.2%), remained stable in 71 (55.5%) and worsened in 3 eyes (2.3%), with a maximum increase of 9/10 and a maximum decrease of 8/10.
Visual field improved in 65 eyes (53.3%) and remained stable in 57. In 6 eyes the visual field evaluation could not be carried out.
Baseline iVEPs reliability: accordance to the preoperative vision
Mean BIS value during the recording phase was 47.9 ± 3.2. The baseline iVEPs amplitude strictly correlated with the preoperative visual assessment. In detail, we found a significant correlation between preoperative visual acuity and iVEPs amplitude (p = 0.0010, Spearman correlation coefficient). Mean baseline iVEPs amplitude was significantly higher in patients with preoperative visual acuity > 2/10 than in patients with preoperative visual acuity ≤ 2/10 (2.1 ± 1.8 vs 0.7 ± 0.9, p < 0.0001, Mann–Whitney U test) (Fig. 3, left). Moreover, mean preoperative iVEPs amplitude was significantly higher in patients with normal preoperative visual field than in patient with altered preoperative visual field (2.6 ± 1.4 vs 1.6 ± 1.8, p = 0.0004, Mann–Whitney U test) (Fig. 3, right).
iVEPs and postoperative visual function
iVEPs variations were strongly correlated with the postoperative visual changes. In detail, we found a linear correlation between the changes of visual acuity and changes of VEP amplitude (p < 0.0001, Spearman correlation coefficient). Moreover, mean percent iVEPs variation was significantly higher in patients with improved visual acuity than in patients with stable or worsened visual acuity after surgery, and in patients with improved visual field than in those with stable or worsened visual field after surgery (p < 0.0001 and p = 0.0013, respectively; Mann–Whitney U test; Fig. 4).
Postoperative iVEPs variation and postoperative vision. Left. Box plot comparing iVEPs variation in patients with improved vs not improved postoperative visual acuity. Postoperative iVEPs amplitude significantly increased in the former vs the latter patients (28.7% ± 22.9% vs − 4.9% ± 16.5%, p < 0.0001, Mann–Whitney U test). Right. Box plot comparing postoperative iVEPs variation in patients with improved vs not improved postoperative visual field. Postoperative iVEPs significantly increased in the former vs the latter patients (16.9% ± 26.6% vs 0.7% ± 21.8%, p = 0.0013, Mann–Whitney U test)
Sensitivity, specificity, and predictive value of iVEPs for visual function
By ROC analysis, we found that the percent iVEPs variation was a good predictor of visual acuity improvement after surgery (AUC 0.896), with a best cutoff set at zero (Fig. 5, left). Conversely, ROC analysis showed that the percent iVEPs variation was a suboptimal predictor of visual field improvement after surgery (AUC 0.654): also, in the latter, the best cutoff was set at zero (Fig. 5, right).
We dichotomized the percent iVEPs variation using the identified best cutoff to establish the predictive positive and negative values of iVEPs variation for vision improvement after surgery. As concerns visual acuity improvement, predictive positive value was 91.3% and a predictive negative value was 85% (p < 0.0001, Fisher Exact test; Table 2). By limiting analysis to those patients with preoperative visual acuity < 10/10, we found a positive predictive value of VEP variation for visual improvement of 100%, whereas the negative predictive value dropped at 78.2% (p < 0.0001, Fisher exact test). In other words, an improvement of iVEPs predicts a visual acuity recovery in all cases.
As expected, the positive and negative predictive values of iVEPs variation for visual field improvement were lower (74.4% and 58.2%, respectively, with p = 0.0006; Fisher exact test; Table 2). By limiting the analysis at those patients with preoperative reduced visual field, the positive predictive value raised to 86.5%, while the negative predictive value remained low (42.1%) (p = 0.0055, Fisher Exact Test). In other words, an iVEPs augmentation was associated to a postoperative visual field restitution, and patients with stable or reduce iVEPs could also have the chance of a postoperative visual field improvement.
Discussion
State of the art of techniques and reliability of intraoperative monitoring of VEPs
The VEPs, as widely described, allow an objective comprehensive evaluation of the function of the optic nerve, chiasm, and the whole visual pathway, without the needs of patient’s cooperation [10, 21, 31]. Furthermore, it has been proven that compression of visual pathway, as well as intrinsic lesions of the optic-chiasmatic apparatus, can influence VEPs, resulting in an increase in latency, as well as in a reduction of the amplitude of the wave potential [7, 26, 27, 31].
Nevertheless, conflicting data are reported on the intraoperative reliability of VEPs as predictors of visual outcome. Inconclusive results are probably affected by the inconsistency of anesthetic and monitoring techniques, particularly in older studies. The introduction of total intravenous anesthesia (TIVA) [27, 29, 30] and the simultaneous use of EEG monitoring during iVEPs acquisition [13] allowed more stable and reliable acquisitions during surgery. In Table 3, we resumed the findings of the most numerous clinical series: incompleteness of reported data and the consequent inconclusive results are evident [3, 4, 8, 13, 15, 17,18,19, 22, 23, 28].
Findings of the present study
Realization of a robust and reliable set-up for iVEP monitoring
We obtained a reliable and reproducible iVEP monitoring in all 64 included patients, regardless of clinical characteristics and type of pathology. Our clinical series is homogeneous in terms of neuro-anesthetic (all patients performed TIVA) and neurophysiological protocols (iVEPs, ERG, and EEG), approach (EEA) and surgical team (Neurosurgeon and ENT surgeon).
Notably, our study differs in the stimulation and recording techniques respect to the previous literature. In fact, the light power of the stimulus used in most of the published studies is 2000 lx with a stimulus duration of 20 ms and a stimulation frequency of 1 Hz, while in our study 3 white light LEDs were used with a power of 5500 lx each (total 16,500 lx for each eye) with a stimulus duration of 5 ms and a stimulation frequency of 1 Hz. In our experience, the high brightness and the shortness of the stimulus are important to obtain a recordable response from suffering optical pathways such as those examined; on the other hand, a stimulus of low and prolonged intensity is unlike to evoke recordable responses. Furthermore, most of the published series reported on the use of goggle glasses with red LEDs, which are less effective in retinal stimulation than the white light LEDs used in this study [3, 15, 17, 19, 23, 28]. Finally, in many papers simultaneous ERG monitoring was not carried out [4, 8, 13, 18, 22], which instead is an important parameter for verifying the effectiveness of the VEP visual stimulation performed [2, 9, 11, 15, 23, 24].
Baseline iVEPs values strongly correlate with preoperative visual function
Our results confirm that the basal iVEPs, and in particular the wave amplitude, significantly correlate with the preoperative visual status, both as regards visual acuity and visual field (Fig. 3). Such data confirm the reliability and robustness of the neuroanesthetic/neurophysiological protocols adopted.
Modifications of iVEPs strongly correlate to changes in visual function
Our results testify that stable variations of the iVEPs were strongly correlated to changes in postoperative vision, particularly concerning visual acuity (Fig. 4). Specificity and positive predictive value were remarkable high (94.4% and 91.3%, respectively) (Fig. 5 and Table 3).
An important point is to establish the ideal threshold to consider a stable modification of iVEP amplitude “significant.” In most studies, an iVEP with > 50% increase in amplitude from baseline is generally considered “improved” and an iVEP with > 50% decreased amplitude from baseline is worsened [4, 8, 17, 28]. This relatively high threshold of variation can be justified by the relative instability of the visual potentials or by the difficulty in interpreting more subtle modifications. Few studies used lower thresholds, with unclear results [22]. In the present study, we planned to consider significant any variation of VEP amplitude regardless of its extent (with a threshold set at 10% to account for background noise) but in relation to its stability over time. Our analysis demonstrates that an amplitude augmented of ≥ 10% was able to predict postoperative visual improvement with 100% reliability. On the other hand, we were not able to establish a statistical correlation between the worsening of iVEPs and visual worsening because of the small number of worsening observed: but what we can say is that a reduction of final iVEPs amplitude has been observed only in the 2 patients (3 eyes) that experienced a worsening of the vision post-surgery. One of the patients was harboring a giant and harsh relapsed nonfunctioning pituitary adenoma invading the posterior fossa: in this case removal was complete and worsening was probably due to injury of the vascular supply of the branches of the superior hypophyseal arteries supplying the chiasma; this patient accounted for 2 out of the 3 postoperative visual worsening in this series; the other patient had a stiff tuberculum sellae meningioma that was very strictly adherent to the left optic nerve; in this patient, because of iVEPs deterioration in the left eye during tumor removal, we decided to interrupt surgery since a satisfied decompression had been already obtained.
Strengths and limitations of the present study
We homogeneously and prospectively collected and analyzed data from a single tertiary skull base reference center; moreover, we obtained statistically robust evidence on the positive predictive role of iVEP in predicting patients’ visual status. These considerations build up the strong points of the study.
As limitations, the neurophysiologic and anesthesiologist setting can be somewhat demanding and needs to be validated in multicenter studies to assess generalizability. Moreover, the predictive value of transient iVEPs worsening could not be rigorously assessed (explaining the absence of correlation between mean percent iVEPs variation and visual function in patients with worsened visual acuity after surgery), nor was a correlation between a precise surgical step (dural opening, tumor debulking, and so on) and corresponding iVEPs variation.
It is worth to note that our results were obtained in a particular setting with an EEA and cannot be translated in other surgical approaches. It is in fact our experience that by using the same intraoperative protocol during intervention performed with a transcranial approach the methods can be less reliable due to displacement of stimulating ipsilateral goggle induced by the surgical maneuvers as frontal skin detachment necessary for the flap and craniotomy.
Conclusion
The present study demonstrates that iVEPs can be reliably recorded during EEA. We set up a reproducible method and identified the cutoff values of significance for the variations in amplitude that occur during surgery. Further studies are necessary to demonstrate and confirm the sensitivity of this neurophysiological tool in guiding live surgery.
Abbreviations
- iVEPs:
-
Intraoperative visual evoked potentials
- EEA:
-
Endonasal endoscopic approach
- SSEP:
-
Somatosensory evoked potentials
- MEP:
-
Motor evoked potentials
- VEPs:
-
Visual evoked potentials
- EEG:
-
Electroencephalogram
- ENT:
-
Ear, nose, and throat
- ERG:
-
Electroretinogram
- TIVA:
-
Total IntraVenous Anesthesia
- TCI:
-
Target Controlled Infusion
- BIS:
-
Bispectral index
- ABB:
-
Acid base balance
- CSF:
-
Cerebro-spinal fluid
- SD:
-
Standard deviation
- AUC:
-
Area under the curve
References
CBTRUS 2002 statistical report: primary brain tumors in the United States, 1995–1999. Central Brain Tumor Registry of the United States. Available from: http://www.cbtrus.org
Cedzich C, Schramm J, Fahlbusch R (1987) Are flash-evoked visual potentials useful for intraoperative monitoring of visual pathway function? Neurosurgery 21(5):709–715
Chacko AG, Babu KS, Chandy MJ (1996) Value of visual evoked potential monitoring during trans-sphenoidal pituitary surgery. Br J Neurosurg 10(3):275–278
Chung SB et al (2012) Intraoperative visual evoked potential has no association with postoperative visual outcomes in transsphenoidal surgery. Acta Neurochir (Wien) 154(8):1505–1510
D’alessandris QG, Rigante M, Mattogno PP, La Rocca G, Romanello M, Auricchio AM, Bevacqua G, Fraschetti F, Giordano M, Di Bonaventura R, Pallini R, Anile C, Olivi A, Lauretti L (2020) Impact of 4K ultra-high definition endoscope in pituitary surgery: analysis of a comparative institutional case series. J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.20.04875-4
Doglietto F, Prevedello DM, Jane JA Jr, Han J, Laws ER Jr (2005) Brief history of endoscopic transphenoidal surgery–from Philipp Bozzini to the first world congress of endoscopic skull base surgery. Neurosurg Focus. 19(6):E3 (15)
Feinsod M, Selhorst JB, Hoyt WF, Wilson CB (1976) Monitoring optic nerve function during craniotomy. J Neurosurg 44(1):29–31
Feng R, Schwartz J, Loewenstern J, Kohli K, Lenina S, Ultakan S, Iloreta AM, Govindaraj S, Bederson J, Banik R, Shrivastava R (2019) The predictive role of intraoperative visual evoked potentials in visual improvement after endoscopic pituitary tumor resection in large and complex tumors: description and validation of a method. World Neurosurg. 126:e136–e143. https://doi.org/10.1016/j.wneu
Flanagan J, Harding G (1988) Multi-channel visual evoked potentials in early compressive lesions of the chiasm. Doc Ophthalmol 69(3):271–281
Gott PS, Weiss MH, Apuzzo M, Van Der Meulen JP (1979) Checkerboard visual evoked response in evaluation and management of pituitary tumors. Neurosurgery 5(5):553–558
Gutzwiller EM et al (2018) Intraoperative monitoring with visual evoked potentials for brain surgeries. J Neurosurg 130(2):654–660
Hadad G, Bassagasteguy L, Carrau RL et al (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116:1882–1886
Houlden DA et al (2014) Intraoperative flash VEPs are reproducible in the presence of low amplitude EEG. J Clin Monit Comput 28(3):275–285
Jashek-Ahmed F, Cabrilo I, Bal J, Sanders B, Grieve J, Dorward NL, Marcus HJ (2021) Intraoperative monitoring of visual evoked potentials in patients undergoing transsphenoidal surgery for pituitary adenoma: a systematic review. BMC Neurol 21(1):287 (23)
Kamio Y, Sakai N, Sameshima T et al (2014) Usefulness of intraoperative monitoring of visual evoked potentials in transsphenoidal surgery. Neurol Med-Chir 54(8):606–611
Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K (2010) Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir (Wien) 152:643–648
Kurozumi K et al (2017) Simultaneous combination of electromagnetic navigation with visual evoked potential in endoscopic transsphenoidal surgery: clinical experience and technical considerations. Acta Neurochir (Wien) 159(6):1043–1048
Luo Y et al (2015) Clinical utility and limitations of intraoperative monitoring of visual evoked potentials. PLoS One 10(3):e0120525
Nishimura F et al (2018) Efficacy of the visual evoked potential monitoring in endoscopic transnasal transsphenoidal surgery as a real-time visual function. Neurol India 66(4):1075–1080
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A et al (2016) ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol 133:1–9
Petersen J (1984) Objective determination of visual acuity by visual evoked potentials. Spec Tests Vis Funct 9:108–114
Qiao N, Song M, Ye Z, He W, Ma Z, Wang Y, Zhang Y, Shou X (2019) Deep learning for automatically visual evoked potential classification during surgical decompression of sellar region tumors. Transl Vis Sci Technol 8(6):21.141414
Sasaki T et al (2010) Intraoperative monitoring of visual evoked potential: introduction of a clinically useful method. J Neurosurg 112(2):273–284
Sato A (2016) Interpretation of the causes of instability of flash visual evoked potentials in intraoperative monitoring and proposal of a recording method for reliable functional monitoring of visual evoked potentials using a light-emitting device. J Neurosurg 125(4):888–897
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, Youngs EJ (1998) The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 88(5):1170–1182
Semela L, Hedges TR, Vuong L (2007) Serial multifocal visual evoked potential recordings in compressive optic neuropathy. Ophthalmic Sur Lasers Imaging Retina 38(3):250–253
Tanaka R, Tanaka S, Ichino T, Ishida T, Fuseya S, Kawamata M (2020) Differential effects of sevoflurane and propofol on an electroretinogram and visual evoked potentials. J Anesth 34(2):298–302. https://doi.org/10.1007/s00540-020-02733-7
Toyama K, Wanibuchi M, Honma T, Komatsu K, Akiyama Y, Mikami T, Mikuni N (2020) Effectiveness of intraoperative visual evoked potential in avoiding visual deterioration during endonasal transsphenoidal surgery for pituitary tumors. Neurosurg Rev 43(1):177–183
Uribe AA et al (2017) Comparison of visual evoked potential monitoring during spine surgeries under total intravenous anesthesia versus balanced general anesthesia. Clin Neurophysiol 128(10):2006–2013
Wiedemayer H et al (2003) Visual evoked potentials for intraoperative neurophysiologic monitoring using total intravenous anesthesia. J Neurosurg Anesthesiol 15(1):19–24
Wilson W, Kirsch W, Neville H, Stears J, Feinsod M, Lehman R (1976) Monitoring of visual function during parasellar surgery. Surg Neurol 5(6):323–329
Zada G, Du R, Laws ER Jr (2011) Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg 114(2):286–300
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Prot. 17814/19, ID: 2557
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
In this study, Mattogno et al. presented a series of 64 patients who underwent endoscopic transphenoidal surgery for pituitary adenomas with intraoperative visual evoked potentials (iVEPs).The authors have refined the iVEP acquisition by using a novel stimulation technique, obtaining good correlations with the clinical outcome. This is an additional step forward in the evolution of iVEPs over the past fifteen years. iVEPs were largely abandoned for many decades due to technical limitations, the lack of electroretinogram recording and the fact that, being a polysynaptic pathway, it is very sensitive to general anesthesia. Most of these limitations have been overcome and nowadays iVEPs are increasingly used in Neurosurgery. Additional series, like this from Mattogno et al., will contribute to understand the real value of iVEPs in predicting and, foremost, preventing injury to visual pathways during various neurosurgical procedures.
Francesco Sala
Verona, Italy
Pier Paolo Mattogno and Quintino Giorgio D’Alessandris are equal contributors. Alessandro Olivi and Liverana Lauretti are co-senior authors.
This article is part of the Topical Collection on Pituitaries.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mattogno, P.P., D’Alessandris, Q.G., Rigante, M. et al. Reliability of intraoperative visual evoked potentials (iVEPs) in monitoring visual function during endoscopic transsphenoidal surgery. Acta Neurochir 165, 3421–3429 (2023). https://doi.org/10.1007/s00701-023-05778-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05778-1