Abstract
Purpose
With the increasing role of molecular genetics in the diagnostics of intracranial tumors, delivering sufficient representative tissue for such analyses is of paramount importance. This study explored the rate of successful diagnosis after frame-based stereotactic biopsies of intracranial lesions.
Methods
Consecutive patients undergoing frame-based stereotactic biopsies in 2020 and 2021 were included in this retrospective analysis. Cases were classified into three groups: conclusive, diagnosis with missing molecular genetics (MG) data, and inconclusive neuropathological diagnosis.
Results
Of 145 patients, a conclusive diagnosis was possible in n = 137 cases (94.5%). For 3 cases (2.0%), diagnosis was established with missing MG data. In 5 cases (3.5%), an inconclusive (tumor) diagnosis was met. Diagnoses comprised mainly WHO 4 glioblastomas (n = 73, 56%), CNS lymphomas (n = 23, 16%), inflammatory diseases (n = 14, 10%), and metastases (n = 5, 3%). Methylomics were applied in 49% (n = 44) of tumor cases (panel sequencing in n = 28, 30% of tumors). The average number of specimens used for MG diagnostics was 5, while the average number of specimens provided was 15. In a univariate analysis, insufficient DNA was associated with an inconclusive diagnosis or a diagnosis with missing MG data (p < 0.001). Analyses of planned and implemented trajectories of cases with diagnosis with missing MG data or inconclusive diagnosis (n = 8) revealed that regions of interest were reached in almost all cases (n = 7).
Conclusion
Although stereotactic frame-based biopsies deliver a limited amount of tissue, they bear high histopathological and molecular genetic diagnostic yields. Given the proven surgical precision of the planned biopsy trajectories, optimizing surveyed lesion regions could help improve the rate of conclusive diagnoses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stereotactic biopsies are an established modality for the diagnostic workup of unclear, deep-seated intracranial lesions. This mostly includes lesions suspicious of malignant tumors that cannot be resected in an oncologically sound or functionally safe manner [4]. Paradoxically, being a minimally invasive surgical modality with very low morbidity, the advantage of stereotactic biopsies can at the same time be its very disadvantage. For non-resectable, eloquent, multifocal, and deep-seated intracranial lesions, stereotactic frame-based biopsies can deliver only a finite amount of tissue for neuropathology studies [10]. This can lead to, albeit rarely [6], an inconclusive diagnosis.
While in the past, classic histopathology and immunohistochemistry have been applied in the analysis of biopsy specimen, next-generation sequencing (NGS) and methylation studies have revolutionized the diagnostics of brain tumors in the past. After the updated World Health Organization (WHO) classification of central nervous system (CNS) tumors in 2021, which integrates molecular and histopathological tumor characteristics [11], molecular analyses have indeed become an essential part of the neuropathology workup. In part, this reflects that neuro-oncology is increasingly driven by individualized therapy concepts [2, 27]. However, besides molecular targets, also basic classification often depends on molecular parameters [11]; an emerging role of stereotactic biopsies would not only entail providing sufficient tissue for a viable diagnosis including molecular biomarkers, but also for studies that are translated into personalized targeted therapy for affected patients [5, 11].
In the wake of more complex neuropathology analyses, it would be reasonable to investigate whether the tissue material delivered under current stereotactic biopsy conditions is sufficient to fulfill new diagnostic standards. This study hence aimed to provide an insight into the diagnostic yield of stereotactic frame–based biopsies of intracranial lesions in a high-throughput comprehensive neuro-oncology center with a special focus on tissue molecular genetics. It then explores whether possible surgical factors can be optimized to prevent the delivery of material yielding unsuitable DNA for molecular profiling through stereotactic biopsies and therefore hamper the compilation of an “integrated diagnosis.”
Methods
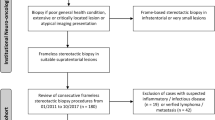
In this retrospective study, clinical and histopathological data of all consecutive patients undergoing frame-based stereotactic biopsies for an unclear intracranial lesion in the years 2020 and 2021 at our neurosurgical department were included.
Surgical procedure
Patients were operated under general anesthesia. After having placed the stereotactic frame, intraoperative computer tomography or magnetic resonance imaging (MRI) imaging was performed to either plan the desired trajectory or fuse it with preoperative images that obtained the stereotactic plan. Biopsies were performed with the stereotactic system (Zamorano-Duchovny or Riechert-Mundinger, inomed Medizintechnik, Emmendingen, Germany) using a guided biopsy forceps (inomed Medizintechnik GmbH, Emmendingen, Germany) via a burr hole trephination placed in line with the trajectory. Specimens were taken in a serial fashion each millimeter from the border of the lesion (labeled X mm) to the planned target point within the lesion (labeled ± 0 mm).
Tissue and image analysis
The overall number of specimen as well as the number used for histological and molecular neuropathology were analyzed. Molecular neuropathological studies included: immunohistochemistry, 850 k methylomics array described under [2], panel sequencing, i.e., customized NGS gene panel capturing the entire coding and selected intronic and promoter regions of 130 genes altered in CNS tumors informing on single nucleotide variations, fusions, and copy number aberrations [21], and RNA sequencing, i.e., next-generation mRNA sequencing [24]. Depending on the final diagnosis in the neuropathology report, cases were assigned to one of the following groups: (1) conclusive, (2) diagnosis with missing molecular genetic data, and (3) inconclusive neuropathological diagnosis.
Pre- and postoperative MRI scans and stereotactic trajectory planning images were analyzed in a subset of cases (inconclusive cases and cases with insufficient isolated DNA for MG). Firstly, post-operative MRI scans were utilized to analyze with trajectories classified into optimal and sub-optimal, depending on their overlap with the region of interest within the tumor (contrast enhancement, fluid attenuated inversion recovery (FLAIR), and necrosis). The positions of numbered specimens were then matched with the specific specimens used for tissues sequencing by the neuropathology department.
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range, while ordinal and nominal variables are presented as numbers and frequencies. Comparison of nominal variables between groups was performed using chi-Square or Fisher’s exact test (depending on group size) or non-paired, double-tailed Student’s t-test (in paired samples and independent samples). Significance was deemed to be reached at p < 0.05 and all statistical analyses were performed using GraphPad PRISM (Version 9; GraphPad Software Inc., Boston, MA; USA).
Results
Frame-based stereotactic biopsies bear a sufficient diagnostic yield
In this cohort, a total of 145 consecutive patients undergoing frame-based stereotactic biopsies in 2020 and 2021 as part of a workup for unclear intracranial lesions were included. This cohort (n = 80, 55% males) had an average age of 58.3 years (SD ± 20.2 years). Most lesions were deep-seated (thalamus/basal ganglia, 20.1%, corpus callosum 17%) or in eloquent regions of the frontal or temporal lobes (19% and 13%, respectively). Eight percent of the biopsied lesions were infratentorial. Overall, 8% of the patients included showed multifocal lesions. Preoperatively, by means of clinical and radiological features, suspected diagnosis mostly included glioma (66%) and lymphoma (20%). This is also reflected by the radiographic features of these lesions, with contrast enhancement in 85% and FLAIR hyperintensity without contrast enhancement found in the remaining 15% of the cases (see Table 1). Further suspected diagnoses included inflammatory diseases in 8% of the cases and metastasis in 3%. In four cases, pretreated patients were biopsied with the suspicion of tumor recurrence after a tumor board recommendation for tissue sampling with the possibility of personalized therapy.
After stereotactic frame-based biopsy, the final diagnosis groups in the neuropathology report included glioma in 92 cases (63%), intracranial lymphoma in 23 cases (16%), inflammatory disease in 14 cases, and metastases in five cases (all suspected cases confirmed). A net number of 13 cases (9%) showed a cross-over, i.e., the suspected diagnosis constituted a different entity to the final diagnosis, with most cross-over cases entailing lymphomas to glioma (and less so vice versa, see Figs. 1 and 2).
Most diagnosed glioma cases included isocitrate dehydrogenase (IDH)-wildtype glioblastoma (GBM), WHO grade 4 (n = 73, 79% of all gliomas). IDH-mutant astrocytoma was diagnosed in 11 cases (WHO grade 2 to 4), and IDH-mutant, 1p-19q co-deleted oligodendrogliomas in only 2 cases (see Fig. 2). Across all 92 glioma cases, microscopy along with immunohistochemistry and O6-methylguanine-DNA-methyltransferase (MGMT) pyrosequencing was sufficient for a final, integrative diagnosis in 45 cases (49% of the total glioma subset). DNA isolation for methylome analysis was performed in 44 cases (48% of glioma cases). In three of these cases (3% of all diagnosed gliomas), a low amount of DNA was isolated from tissue specimens, whereas in 41 cases, enough DNA was isolated. In a subset of cases where methylation analyses were applied (n = 21, 22% of glioma patients), further analyses including next-generation DNA panel sequencing and RNA sequencing (RNA-Seq) were also performed (Fig. 1). From this further subset, only three cases with a low amount of DNA hindered the attainment of a final diagnosis. In total, 89 glioma cases (97%) constituted a complete integrated diagnosis, and only 3 glioma cases remained with missing molecular genetic data due to a low amount of isolated DNA. Two of these cases were then diagnosed as “malignant glioma” (as mentioned in the neuropathology official report). In the third case, BRAF sequencing could not be carried out in an IDH-mutant glioma case, because tumor DNA had already been used up for methylome analysis.
In further 5 cases from the study cohort (3% of all cases), an inconclusive diagnosis was provided. In 3 of the 5 cases, this was attributed the fact that further analyses were not possible due to a low amount of isolated DNA for molecular genetics. In total, eight cases (n = 3 glioma with insufficient molecular genetics (MG) and n = 5 inconclusive diagnosis) did not show a fully conclusive diagnosis, with insufficient DNA isolated in 6 of these 8 cases. Of note, only one of the 5 inconclusive cases emerged as a GBM in a 3-month follow-up. Interestingly, in 2 cases of conclusive diagnosis (one case of glioblastoma, IDH-wildtype, and a further case of inflammatory disease), a disclaimer was issued over quality of the data due to “low DNA amount.” In total, eight samples in the study cohort with a low mount of DNA were identified (see Table 2).
Cases with inflammatory disease
One case of toxoplasmosis, three cases of CNS vasculitis, two cases of encephalitis, and three further cases of inflammatory demyelinating diseases. In the remaining five cases, CNS tissue with reactive changes was the official final diagnosis.
Surgical complications
Surgical complications included one non-surgically relevant hemorrhage along the biopsy trajectory, and a single case of surgically revised wound healing disorder, setting the surgical complication rate of stereotactic biopsies in this cohort at 1%.
Adjuvant therapies
After successful diagnosis of 92 gliomas, 23 lymphomas, and 5 metastases (a total of 120 tumors), 81 (67.5%) patients received chemotherapy and 74 (62%) received postoperative radiotherapy. Of all 92 glioma cases, four patients (4%) received an off-label non-standard of care systemic therapy, based on tissue material from stereotactic biopsies.
Insufficient DNA material is not a result of a low number of specimens or inaccurate trajectories
To eliminate possible surgical reasons for insufficient DNA material for sequencing or inconclusive diagnoses, we thoroughly examined the previously introduced eight cases. Firstly, we compared the number of specimens delivered in these eight cases with the rest of the patient cohort. Across all patients, an average number of 15.74 specimens were taken (SD, 4.46 specimens). There was no statistically significant difference in number of specimens taken in conclusive (n = 138) versus other cases (n = 8, glioma with insufficient MG and inconclusive). The average number of specimens in the conclusive group was 15.76 (SD 4.5) vs. an average of 15.5 with a standard deviation of 3.7 in a group containing all other cases (p = 0.86, non-paired t-test, Fig. 2). As expected, missing DNA was associated with an inconclusive diagnosis (n = 3/5) or a diagnosis with missing MG data (n = 3/5, total n = 6/8) vs n = 2/137 in the conclusive diagnosis group (p < 0.001, Fisher’s exact test). Because FLAIR lesions are usually less circumscribed on MRI images and can be an expression of a variety of pathologies besides tumors, we determined whether FLAIR lesions were overrepresented in cases without conclusive diagnoses compared to contrast-enhancing lesion. However, differences were non-significant (2/23 FLAIR lesions, p = 0.6131, Fisher’s exact test, see Table 2).
After ruling out that the quantity of delivered specimens was associated with incomplete diagnoses in these 8 cases, we then analyzed the surgically expected “quality” of the specimens. All planned trajectories in these 8 cases were assessed as to (1) whether these trajectories were correctly biopsied based on image fusion of postoperative MRI scans with the planned trajectory (whenever available); (2) in which cases, although potentially less relevant, the number of biopsy specimens could have been maximized based on the radiographic feature of the lesion; and (3) whether the planned trajectories represented the “relevant areas” of the lesion (contrast-enhancement, FLAIR) and how this in turn corresponded to the specimens sequenced by neuropathology (Fig. 3).
Trajectory analysis of cases with missing MG data and inconclusive diagnosis (n = 8). Samples were taken as serial biopsies with each specimen being taken at a 1-mm interval. Specimens were numbered with negative values in relation to their distance in millimeters to the planned final specimen (“0”)
In all eight cases, postoperative MRI scans were available and showed that the biopsies were carried out along the planned trajectories (100%). With regard to the number of specimens, in five of the eight cases, more specimens along the planed trajectory could have hypothetically been acquired. The lesions of interested were contrast-enhanced in six cases and FLAIR-hyperintense in two cases. Qualitative analyses of whether the planed trajectories overlap with the region of interest of the lesion revealed that in seven of the eight cases the trajectory was, in opinion of the surgeon performing the biopsy, sufficient to survey the relevant parts of the suspected tumor. In all seven cases with optimal trajectories, specimens used for sequencing analyses originated from the regions of interest. Only in one case (SN 82, see Table 3) the stereotactic trajectory was retrospectively considered nonoptimal (biopsy of mostly the necrotic parts of the lesion), which is why sequencing from the contrast enhancing region of the lesion was not possible.
Discussion
The increasing complexity of neuropathology reports has been observed by all stakeholders involved in neuro-oncology patient care. In addition to light microscopy and immunohistochemistry, an arsenal of methods including MGMT-pyrosequencing, methylome profiling, panel sequencing, and in rare cases RNA sequencing has become pivotal in providing not only an integrated histopathological and molecular diagnosis, but also further data on tumor epigenetics, transcriptomics, and metabolomics that is becoming increasing relevant to therapy and prognosis. Corresponding to this, the application of targeted tumor therapies is observed in neuro-oncology, either within clinical trials or under off-label conditions especially in progressive or recurrent tumors. At the same time and in frequent cases where tumor recurrence is suspected, ruling out post-therapeutic differential diagnosis like radiation necrosis via stereotactic biopsy surgery could help avoid more extensive forms of respective repeat surgery, especially in patients with tumor-associated reduced status. With this, stereotactic neurosurgery faces the challenge of delivering quantitatively and qualitatively sufficient tissue to meet such expectations [26].
In this analysis, 97% of the cases a sufficient diagnosis was met through stereotactic biopsies. This included the provision of adequate material for analyses beyond what is needed for an “integrated diagnosis.” Indeed, in most cases where isolation of tissue material was needed for methylome profiling, panel sequencing, or RNA sequencing, there was sufficient material to enable this workup. Only in three cases (3% of gliomas), a suspected glioma fell short of an integrated diagnosis due to insufficient DNA material. Overall, in 3% of cases in this cohort, an inconclusive diagnosis was met.
Similar rates for success of stereotactic biopsies in providing a clinically sufficient diagnosis have been reported in previous studies [15, 25]. With molecular data in focus, a study on specifically diffuse intrinsic pontine glioma pediatric patients in 2019 showed lower rates, even when including open biopsies and sub-total resections likely providing more tissue compared to a stereotactic biopsy [18]. At the same time, neuropathological tissue processing methods could be stream-lined at the possible cost of needing more tissue in the future, for example through nano-pore sequencing [14, 17]. In another study with a similar rate of conclusive diagnosis, the rate of applied methylome analyses was lower (44 cases within a 5-year period compared to 44 cases at our center in one year). In a center with neuro-oncology focus, center bias could indeed explain this high rate of methylome studies on unclear intracranial lesions. Therefore, it must be noted that although methylation analyses were performed in these cases, it is unclear in what percentage thereof a final diagnosis would have been possible without such analysis. However, the purpose of the study was to merely demonstrate that such studies, if needed, are almost always feasible under the status quo.
In an interesting observation, a relevant cross-over was observed between suspected intracranial lymphomas and gliomas (and vice versa). In the light of their possible effect on the reliability of the diagnosis [16], delaying admission of corticosteroids until after the biopsy has a direct influence on the clinical management of affected patients, although this notion is currently challenged [22, 23]. Optimization of imaging modalities might help in diminishing this phenomenon [7, 12, 13]. The surgical complication rate in this cohort was, in line with previous studies, very low (< 1%) [20].
In terms of the critical number of specimens for sufficient sequencing workup, this study found that in average, five specimens are needed. This number seems reasonable and feasible, considering the average number of taken specimens in this study (15 to 16, SD of 4). As shown by the data, the number of specimens in general did not present a challenge for conclusive diagnosis, and as expected, a low DNA amount in biopsy probe is associated with an inconclusive diagnosis. The idea that non-contrast enhancing lesions (FLAIR-hyperintense lesions) are “more susceptible” to inconclusive diagnosis was not proven by the data. In the case of FLAIR lesions, established supportive modalities like Positron emission tomography scans are known to improve the visualization of “nodular/cell-rich” and metabolically active regions within the lesion [1, 19].
Only four patients in this cohort underwent stereotactic biopsies for recurrent glial tumors due to progressive disease that were beyond feasible resection options with the specific question of providing tissue material for targeted therapy or ruling out pseudo-progression. The rate of such interventions is projected to increase in the future. The data presented in this analysis, along with similar study results on gliomas and metastases, show that even in this setting, stereotactic biopsies would fulfill expectations [8, 9]. In the wake of the age of precision oncology, it would be reasonable to speculate that more tissue material would be required to fulfill standard of emerging complex neuropathological tests. However, bearing technological advances in mind, it could be equally legitimate to argue that less tumor material is needed due to more efficient diagnostic modalities. According to the findings of the study and in a setting beyond the current state, the concern that “more tissue is needed” than what stereotactic biopsies can provide could not be substantiated. In fact, compared with an earlier study with 500 stereotactic biopsies without MG analysis deeply implemented into the neuropathological workflow, neither the rate of conclusive diagnosis (96.8%) nor the average number of taken specimens (n = 16) [3, 28] differed from the results of this analysis. Especially if evaluating stereotactic biopsy against repeat surgery in the setting of a recurrent tumor, a less invasive intervention could be a more viable option, especially with the usually heavily pretreated status of these patients and the peri-operative risk this entails taken into consideration [3, 28].
There are, however, several limitations to this retrospective monocentric study. Firstly, because stereotactic biopsies are known to have an excellent diagnostic yield, an inconclusive diagnosis is a rather rare event. Therefore, a larger patient cohort would have provided more robust data. On the other hand, because the event of DNA isolation was frequent in this cohort (in about 50% of glioma cases), the cohort size is satisfactory and is in general adequate to meet conclusions as to whether in such cases, sufficient DNA material was available. It could be however prudent to study a higher number of patients in order to analyze a larger subset with inconclusive diagnosis and diagnosis with missing MG data, in order to rule out systematic surgical sources of error in a more reliable fashion. Secondly, it is not entirely clear what consequence missing MG data had with respect to the clinical management of affected cases.
In conclusion, although stereotactic frame-based biopsies deliver a finite amount of tissue, they bear an excellent histopathological and molecular genetic diagnostic yield, with rare cases of missing molecular data or rarely inconclusive diagnosis. An optimal trajectory was chosen in almost all inconclusive cases, with DNA isolation from relevant specimens of “relevant” tumor regions. Therefore, none of the surgical variables examined in this study could have been improved to systemically improve the results of this analysis. Therefore, that within the limits of the interpretation of the data presented in this study, in the rare case of insufficient DNA material after stereotactic biopsies, this is not attributable to systematic or surgical aspects.
Data availability
Data is available upon reasonable request.
References
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougère C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC (2016) Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18:1199–1208. https://doi.org/10.1093/neuonc/now058
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Müller HL, Rutkowski S, von Hoff K, Frühwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu CM, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474. https://doi.org/10.1038/nature26000
Chang SM, Parney IF, Mcdermott M, Barker FG, Schmidt MH, Huang W, Laws ER, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 98:1175–1181. https://doi.org/10.3171/jns.2003.98.6.1175
Chen CC, Hsu PW, Erich Wu TW, Lee ST, Chang CN, Wei KC, Chuang CC, Wu CT, Lui TN, Hsu YH, Lin TK, Lee SC, Huang YC (2009) Stereotactic brain biopsy: single center retrospective analysis of complications. Clin Neurol Neurosurg 111:835–839. https://doi.org/10.1016/j.clineuro.2009.08.013
Ghiaseddin A, Hoang Minh LB, Janiszewska M, Shin D, Wick W, Mitchell DA, Wen PY, Grossman SA (2020) Adult precision medicine: learning from the past to enhance the future. Neuro-Oncol Adv 3. https://doi.org/10.1093/noajnl/vdaa145
Hall WA (1998) The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 82:1749–1755. https://doi.org/10.1002/(sici)1097-0142(19980501)82:9%3c1756::aid-cncr23%3e3.0.co;2-2
Jian J, Li Y, Xia W, He Z, Zhang R, Li H, Zhao X, Zhao S, Zhang J, Cai S, Wu X, Gao X, Qiang J (2022) MRI-based multiple instance convolutional neural network for increased accuracy in the differentiation of borderline and malignant epithelial ovarian tumors. J Magn Reson Imaging 56:173–181. https://doi.org/10.1002/jmri.28008
Katzendobler S, Do A, Weller J, Dorostkar MM, Albert NL, Forbrig R, Niyazi M, Egensperger R, Thon N, Tonn JC, Quach S (2022) Diagnostic yield and complication rate of stereotactic biopsies in precision medicine of gliomas. Front Neurol 13:822362. https://doi.org/10.3389/fneur.2022.822362
Katzendobler S, Do A, Weller J, Rejeski K, Dorostkar MM, Albert NL, Forbrig R, Niyazi M, Egensperger R, Tonn JC, von Baumgarten L, Quach S, Thon N (2022) The value of stereotactic biopsy of primary and recurrent brain metastases in the era of precision medicine. Front Oncol 12:1014711. https://doi.org/10.3389/fonc.2022.1014711
Krieger MD, Chandrasoma PT, Zee CS, Apuzzo ML (1998) Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol 14:13–25. https://doi.org/10.1002/(sici)1098-2388(199801/02)14:1%3c13::aid-ssu3%3e3.0.co;2-5
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Malikova H, Koubska E, Weichet J, Klener J, Rulseh A, Liscak R, Vojtech Z (2016) Can morphological MRI differentiate between primary central nervous system lymphoma and glioblastoma? Cancer Imaging 16:40. https://doi.org/10.1186/s40644-016-0098-9
McAvoy M, Prieto PC, Kaczmarzyk JR, Fernández IS, McNulty J, Smith T, Yu K-H, Gormley WB, Arnaout O (2021) Classification of glioblastoma versus primary central nervous system lymphoma using convolutional neural networks. Sci Rep 11:15219. https://doi.org/10.1038/s41598-021-94733-0
Mimosa ML, Al-Ameri W, Simpson JT, Nakhla M, Boissinot K, Munoz DG, Das S, Feilotter H, Fattouh R, Saleeb RM (2022) A novel approach to detect IDH point mutations in gliomas using nanopore sequencing: test validation for the clinical laboratory. J Mol Diagn. https://doi.org/10.1016/j.jmoldx.2022.12.001
Neumann JO, Campos B, Younes B, Jakobs M, Jungk C, Beynon C, Deimling AV, Unterberg A, Kiening K (2018) Frame-based stereotactic biopsies using an intraoperative MR-scanner are as safe and effective as conventional stereotactic procedures. PLoS One 13:e0205772. https://doi.org/10.1371/journal.pone.0205772
Önder E, Arıkök AT, Önder S, Han Ü, Sorar M, Kertmen H, Yılmaz ED, Fesli R, Alper M (2015) Corticosteroid pre-treated primary CNS lymphoma: a detailed analysis of stereotactic biopsy findings and consideration of interobserver variability. Int J Clin Exp Pathol 8:7798–7808
Patel A, Dogan H, Payne A, Krause E, Sievers P, Schoebe N, Schrimpf D, Blume C, Stichel D, Holmes N, Euskirchen P, Hench J, Frank S, Rosenstiel-Goidts V, Ratliff M, Etminan N, Unterberg A, Dieterich C, Herold-Mende C, Pfister SM, Wick W, Loose M, von Deimling A, Sill M, Jones DTW, Schlesner M, Sahm F (2022) Rapid-CNS(2): rapid comprehensive adaptive nanopore-sequencing of CNS tumors, a proof-of-concept study. Acta Neuropathol 143:609–612. https://doi.org/10.1007/s00401-022-02415-6
Pfaff E, El Damaty A, Balasubramanian GP, Blattner-Johnson M, Worst BC, Stark S, Witt H, Pajtler KW, van Tilburg CM, Witt R, Milde T, Jakobs M, Fiesel P, Frühwald MC, Hernáiz Driever P, Thomale UW, Schuhmann MU, Metzler M, Bochennek K, Simon T, Dürken M, Karremann M, Knirsch S, Ebinger M, von Bueren AO, Pietsch T, Herold-Mende C, Reuss DE, Kiening K, Lichter P, Eggert A, Kramm CM, Pfister SM, Jones DTW, Bächli H, Witt O (2019) Brainstem biopsy in pediatric diffuse intrinsic pontine glioma in the era of precision medicine: the INFORM study experience. Eur J Cancer 114:27–35. https://doi.org/10.1016/j.ejca.2019.03.019
Pirotte B, Goldman S, Brucher JM, Zomosa G, Baleriaux D, Brotchi J, Levivier M (1994) PET in stereotactic conditions increases the diagnostic yield of brain biopsy. Stereotact Funct Neurosurg 63:144–149. https://doi.org/10.1159/000100306
Riche M, Amelot A, Peyre M, Capelle L, Carpentier A, Mathon B (2021) Complications after frame-based stereotactic brain biopsy: a systematic review. Neurosurg Rev 44:301–307. https://doi.org/10.1007/s10143-019-01234-w
Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D, Capper D, Koelsche C, Korshunov A, Wiestler B, Buchhalter I, Milde T, Selt F, Sturm D, Kool M, Hummel M, Bewerunge-Hudler M, Mawrin C, Schüller U, Jungk C, Wick A, Witt O, Platten M, Herold-Mende C, Unterberg A, Pfister SM, Wick W, von Deimling A (2016) Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131:903–910. https://doi.org/10.1007/s00401-015-1519-8
Scheichel F, Marhold F, Pinggera D, Kiesel B, Rossmann T, Popadic B, Woehrer A, Weber M, Kitzwoegerer M, Geissler K, Dopita A, Oberndorfer S, Pfisterer W, Freyschlag CF, Widhalm G, Ungersboeck K, Roessler K (2021) Influence of preoperative corticosteroid treatment on rate of diagnostic surgeries in primary central nervous system lymphoma: a multicenter retrospective study. BMC Cancer 21:754. https://doi.org/10.1186/s12885-021-08515-y
Scheichel F, Pinggera D, Popadic B, Sherif C, Marhold F, Freyschlag CF (2022) An update on neurosurgical management of primary CNS lymphoma in immunocompetent patients. Front Oncol 12. https://doi.org/10.3389/fonc.2022.884724
Stichel D, Schrimpf D, Casalini B, Meyer J, Wefers AK, Sievers P, Korshunov A, Koelsche C, Reuss DE, Reinhardt A, Ebrahimi A, Fernández-Klett F, Kessler T, Sturm D, Ecker J, Milde T, Herold-Mende C, Witt O, Pfister SM, Wick W, Jones DTW, von Deimling A, Sahm F (2019) Routine RNA sequencing of formalin-fixed paraffin-embedded specimens in neuropathology diagnostics identifies diagnostically and therapeutically relevant gene fusions. Acta Neuropathol 138:827–835. https://doi.org/10.1007/s00401-019-02039-3
Tanji M, Mineharu Y, Sakata A, Okuchi S, Fushimi Y, Oishi M, Terada Y, Sano N, Yamao Y, Arakawa Y, Yoshida K, Miyamoto S (2023) High intratumoral susceptibility signal grade on susceptibility-weighted imaging: a risk factor for hemorrhage after stereotactic biopsy. J Neurosurg 138:120–127. https://doi.org/10.3171/2022.4.Jns212505
Torp SH, Solheim O, Skjulsvik AJ (2022) The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien) 164:2453–2464. https://doi.org/10.1007/s00701-022-05301-y
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170–186. https://doi.org/10.1038/s41571-020-00447-z
Yang K, Ellenbogen Y, Martyniuk A, Sourour M, Takroni R, Somji M, Gardiner E, Hui K, Odedra D, Larrazabal R, Algird A, Kachur E, Reddy K, Murty N, Farrokhyar F, Singh SK (2022) Reoperation in adult patients with recurrent glioblastoma: a matched cohort analysis. Neurooncol Adv 4:vdac115. https://doi.org/10.1093/noajnl/vdac115
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study concept and design: MJ, OTH. Data collection and analysis: OTH, KA. Data interpretation: OTH, MJ, FS. Writing the manuscript: OTH, MJ, AU. Reviewing and editing: OTH, MJ, FS, AU. Supervision: MJ, AU. All authors approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval
The local standing committee of ethnical practice approved the protocol of this retrospective analysis which has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and for which patient consent was not required.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MJ has received teaching honoraria from inomed Medizintechnik GmbH.
MJ is a consultant for Efinger Instruments GmbH and Co KG.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alhalabi, O.T., Sahm, F., Unterberg, A.W. et al. The molecular diagnostic yield of frame-based stereotactic biopsies in the age of precision neuro-oncology: a cross-sectional study. Acta Neurochir 165, 2479–2487 (2023). https://doi.org/10.1007/s00701-023-05742-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05742-z