Abstract
Introduction
Acute subdural hematoma (aSDH) is one of the main causes of high mortality and morbidity in traumatic brain injury. Prognosis is poor due to the rapid volume shift and mass effect. Cerebral perfusion is likely affected in this condition. This study quantifies perfusion changes in aSDH using early ER polytrauma CT with perfusion imaging (CTP).
Methods
Data of 54 patients with traumatic aSDH were retrospectively collected. Glasgow Coma scale (GCS), perfusion parameters, therapeutic decisions and imaging data including hematoma thickness, midline shift, and hematoma localization were analyzed. The cortical perfusion parameters of each hemisphere, the area anterior to the hematoma (AAH), area below the hematoma (ABH), area posterior to the hematoma (PAH), and corresponding mirrored contralateral regions were determined.
Results
We found a significant difference in Tmax in affected and unaffected whole-hemisphere data (mean 4.0 s vs. 3.3 s, p < 0.05) and a significantly different mean for Tmax in ABH and for the corresponding mirrored area (mABH) (mean 3.8 s vs. 3.1 s, p < 0.05). No significant perfusion changes in cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) were found.
Conclusion
There was a significant elevation of time to maximum (Tmax) values in the underlying cortical area of aSDH. Possible pathophysiological explanations, the influence on immediate surgical decision-making and further therapeutic consequences have to be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) has a raw incidence of 10.1 cases per 100,000 persons for hospitalization [14]. Moderate and severe TBI are one of the main causes for death or lifelong severe impairment, especially in the age population from 29 to 45 [14]. Due to the high variability of trauma mechanisms, different patterns of brain injury are observed at admission in the emergency unit. The native CT scan performed at arrival at the emergency department gives information about acute bleedings, fractures, and volume shifts inside the skull. Many of the primary injury patterns are either directly or indirectly linked to changes in cerebral perfusion. Improving compromised perfusion is one of the main goals in neurological trauma surgery.
The main challenge in decision-making is to decide the overall necessity and urgency of surgical intervention of patients with TBI. Perfusion deficits are thought to play an important role in intracranial bleedings like aSDH, contusion hemorrhage or intracerebral hemorrhage due to the fast volume shifts with compression of brain tissue; these perfusion deficits are at least partly triggered by local or global intracerebral pressure (ICP) increase [10]. CTP assesses overall and local brain perfusion [9, 22]. CBF, CBV, MTT, and Tmax are the main parameters obtained and give information about tissue at risk, local blood flow, and general blood flow. There is a rising field of usability of the mentioned parameters in different perfusion related questions in neurosurgery, for example in chronic subdural hematoma or cranioplasty patients [19, 20], but the relevance for therapeutic decisions in patients with TBI is still unexplained.
There are few data describing perfusion deficits and how relevant these deficits are for therapeutic decisions. Because aSDH is the main determinant in short-time survival in TBI [12], we decide to focus this study on perfusion changes in patients with TBI and therefore aSDH as the leading pathology. For that prospectively collected early perfusion data of patients arriving at our emergency department with severe head trauma and aSDH are retrospectively analyzed.
Methods
We collected data of patients with a traumatic aSDH as leading pathology and available CTP data presenting at our emergency department from 2013 to 2020. CTP is done in our institution as a standard of care for adult patients with suspected TBI and GCS < 8. Exclusion criteria were bilateral aSDH with the same size, imprecise CTP due to patient’s movements, and the absence of perfusion. We collected GCS values, perfusion parameters, laboratory results, therapeutic decisions and imaging data including hematoma width, midline shift, and hematoma localization. The study was approved by the Ethics Committee of the Medical Faculty of the Heinrich-Heine-Universität Düsseldorf (2020–1166).
CTP was performed on Somatom Definition AS 64 Slice CT-Scanner (Siemens Healthineers, Erlangen, Germany) with a saline chaser using 30 mL of contrast medium (Imeron 400, Bracco Imaging, Germany) bolus application with a 5 mL/s flow. Slices from the level of the orbital roof, the external meatus and cella media were selected to measure the perfusion. The perfusion data of the patients were imported to STROKETOOL-CT (Version 2.0, H.-J. Wittsack, http://www.digitalimagesolutions.de). We applied a motion correction to each data set. To estimate the arterial input function, which is the concentration of a contrast medium in an artery measured over time, we chose branches of the anterior and middle cerebral artery which had a clear time-intensity curve. The following calculation with the determined arterial input function of the perfusion parameters produced color maps for CBF, CBV, MTT, and Tmax.
The color maps were converted in numerical values in ECCETAngiotux CT 2 D (ECCET/AngioTux2D: Beck A., Aurich V institute for computer science/university of anatomy Düsseldorf). The regions of interest are marked with a circular border. The border has a width of 1 cm and runs with a distance of 2 mm parallel to the cortex to exclude the outer cerebrospinal fluid spaces. The superior sagittal sinus and the frontal part of the cerebral falx are also excluded. Within the border, the program computed an occipital clockwise rotation to analyze the perfusion parameters in 2-degree steps, which were exported to a table containing 180 regions of interest. It determined CBV, CBF, MTT, and Tmax for each region.
We used the Picture Archiving and Communication System of our institution and native CT images to analyze the angles of the ABH. We used the longitudinal cerebral fissure and frontal crista as a marker for 0-degree occipital and 180 degrees frontal and measured the angle of the ABH. We calculated the degrees for the AAH, PAH and the mirrored degrees (cf. Figure 1). With these values, the median for each parameter for the whole brain, for both hemispheres and for each selected region were calculated.
CBV describes the cerebral blood volume in ml/g and CBF is commonly measured in ml/100 g/min. MTT is defined as \(\frac{CBV}{CBF}\) and is measured in seconds. Tmax is also measured in seconds and describes the time span between the arterial input function and the arrival in brain parenchyma. It can be computed by deconvoluting the arterial input function [5].
Statistics
Statistical analysis was performed in Python (Version 3.9.10). To compare the cerebral perfusion in different areas a two samples independent t-test was used. The significance level was set to \(\alpha =0.05\). The analysis of correlations was performed with the Pearson correlation coefficient.
Results
We collected data of 54 patients with aSDH as leading pathology, their ages ranged from 17 to 92 years and the median age was 60.5 years (Table 1). Aside from aSDH, 31 patients suffered additionally from traumatic subarachnoid hemorrhage, eleven patients showed a contusion hemorrhage, and six patients had an intracerebral hemorrhage. Other concurrent cranial injury patterns were epidural hematoma and fractures of the skull. Forty-six patients had a GCS < 8 and arrived intubated in the emergency unit. Nine patients had an elevated INR due to oral anticoagulation. The main trauma mechanism was falling downstairs (11 patients). Other trauma mechanisms were falls from height, traffic accidents, and accidents with sharp tools.
There was a significant difference in Tmax in affected and unaffected whole-hemisphere data (mean 4.0 s vs. 3.3 s, p < 0.05). There was also a significantly different mean for Tmax in ABH and for the corresponding mABH (mean 3.8 s vs. 3.1 s, p < 0.05) (Table 2). This difference was present for the whole study population and mainly seen in the age group of 40–65 years. An elevated Tmax in ABH in CTP imaging is illustrated in Fig. 2. The Tmax elevation can be visually detected by having the same shape in CTP as the aSDH in native CT scan. No significant perfusion changes in cerebral blood flow (CBF), cerebral blood volume (CBV), and MTT were found (mean CBF 69.7 ml/100 g/s vs. 68.8 ml/100 g/s, p = 0.927; CBV 2.31 ml/100 g vs. 2.29 ml/100 g, p = 0.916; MTT 3.5 s vs. 3.5, p = 0.879). The median values for the whole brain were CBF = 46.1 ml/100 g/s, median CBV = 1.8 ml/100 g, median MTT = 3.38 s, and median Tmax = 4.61 s (Fig. 3). Tmax in the AAH was significantly higher than in the mirrored area for the whole study population (AAH 43.96 s vs. mAAH 34.54 s, p < 0.05). t-test with the PAH and the mirrored area showed no significance. When different lesion patterns were compared — patients with pure aSDH vs. patients with aSDH and other traumatic lesions — no significant difference was found.
There is a moderate but significant correlation between the maximal width of aSDH and Tmax in ABH (r = 0.35, p = 0.0092) and between the level of midline shift and Tmax in ABH (r = 0.41, p = 0.0019). There is no correlation between INR and Tmax in ABH (r = 0.05, p = 0.7188), nor between GCS and Tmax in ABH (r = − 0.23, p = 0.1196).
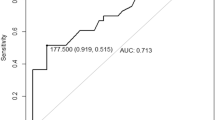
In the examined group, 28 patients were operated, 20 patients were managed conservatively, and 6 patients got a palliative treatment (Table 1). Surgical procedure for hematoma evacuation was either a decompressive craniectomy or, in the case of good primary neurological status, a craniotomy. Patients who underwent surgery had a lower median GCS value (median GCS = 3) than patients who were treated conservatively (median GCS = 5) or palliatively (GCS = 5). The median age of the conservatively managed patients was younger (48.5 years) than the surgically managed patients (59 years), who were younger than the palliatively managed patients (66.5 years). The median thickness of the hematoma was 11.15 mm in the surgery group and 4.25 mm in the conservative group. Conservatively treated patients had a median midline shift of 0 mm, while operated patients had a shift of 5.5 mm. INR was similar in each group (operated 1.15, conservative 1.10, palliative 1.20). In the affected hemisphere, Tmax was significantly higher in patients treated surgically than in patients managed conservatively, whereas the surgical decision was never based on perfusion results (mean Tmax affected hemisphere surgery 4.7 s vs. mean Tmax affected hemisphere conservative 2.7 s, p < 0.001). In addition, we compared Tmax in the affected hemisphere in patients who survived and patients who did not. A difference of the mean values in Tmax (mean Tmax affected hemisphere survival 3.5 s vs. mean Tmax affected hemisphere clinical death 5.2 s, p < 0.05) was detected. The mean Tmax of patients who were treated with palliative care was 5.2 s.
Discussion
CTP is well-known mainly in the setting of acute ischemic stroke and detects the penumbra, which is the tissue in the neighborhood of the acute insult and may be saved by early mechanical or drug-related recanalization therapy [4]. In the DEFUSE-2 study, an elevated Tmax predicted the infarct growth and was a predictive marker for poor outcome, even if the arteries became recanalized [11]. Recent articles have shown promising results using perfusion imaging to diagnose tissue at risk also in the case of traumatic brain injury [2, 23].
In native CT scan, the tissue at risk is hard to delineate and cerebral hematomas like contusions might not be observed at day one [23]. By delineating the tissue at risk in CTP, which is mostly indicated by increased Tmax, early and objective data may possibly contribute to surgical decisions when correlated with clinical aspects. In the setting of severe TBI, different articles point out that not only the primary insult leads to brain damage, but also a secondary ischemic injury related to cerebral edema and local intracranial hypertension [7, 23]. Bendinelli et al. described brain ischemia related to TBI, which is underdiagnosed, but important for decision-making. In his study, CTP data changed the management of three TBI patients with massive brain ischemia that showed only minimal changes in native CT scan [2]. In the present study, operated patients had a higher Tmax and patients selected for palliative care had an extremely elevated Tmax.
Radiologists already use Tmax as an indicator for tissue at risk. Wintermark et al. showed that the detection of tissue at risk is an important prognostic indicator [23]. They included 130 patients with severe TBI and evaluated them with the Glasgow Outcome scale 3 months after injury. The authors found a correlation between higher MTT and a worse outcome and a positive correlation between higher number of arterial territories with high CBV and a better outcome. Furthermore, 18 patients of the study population suffered from aSDH and showed elevated MTT values and lower CBF and CBV values when compared to the control group [23]. In our study, no significant difference in CBV, CBF and MTT was detected, but a higher Tmax was present and correlated to clinical death. No data about long-term outcome are available, but extremely elevated Tmax values ≥ 65 s could only be measured in patients with GCS ≤ 4 and expected very poor outcome. Further, Wintermark et al. compared the perfusion values in different bleedings. The strongest reduction of perfusion was found in patients with an epidural hematoma [23]. Also, intracranial hypertension and cerebral contusions lead to decreased perfusion values. Many studies describe changes in perfusion with CBV, CBF, and MTT in different bleedings, but Tmax is not mentioned [7, 8, 23]. Because of the retrospective design of the current study and the consequential small data set, it was not possible to choose patients with only aSDH. It remains unclear how the mentioned co-pathologies influence the value of Tmax.
Because Tmax is a complex marker and its clinical relevance is still unknown, there is little evidence about its role in TBI. Calamante et al. described Tmax as the arrival delay between the arterial input function and the tissue contrast agent [5]. To calculate Tmax, the arterial input function must be deconvoluted. It is necessary to combine the deconvolution with a regularization to smooth the function. This regularization is influenced by MTT, CBV and the signal-to-noise ratio. For this reason, the deconvoluting algorithm depends on the specific implementation in each institution [5, 21]. In addition to that, CBF, CBV, and MTT are mathematical constructs which are not directly measured and depend on algorithms [21]. Therefore, no generalized norm-values in CTP can be estimated [5, 21], and it is difficult to compare these values as quantitative measurements. For example, CBV may be different in the same patient, because the amount of brain volume in one voxel can differ. In comparison to the other values, Tmax is a time delay-dependent factor detecting a delay of the arrival of contrast bolus in brain tissue. Therefore, it provides relevant additional information about the patient’s hemodynamical status [5]. Several studies mention decreased CBF and CBV and an increased MTT in patients with TBI and SDH [7, 23]. In our study, no significant difference in CBV, CBF and MTT could be detected in the compared brain areas. It is unclear how aSDH affects the brain tissue; therefore, no “healthy” tissue could be defined. Because no normal perfusion values exist, it was not possible to diagnose an elevation or reduction of perfusion values in the whole brain.
The cohort was divided into three groups. As each patient has a different incidence of aSDH, it is not meaningful to compare mean Tmax between groups, but the middle-aged group showed a significant increase in Tmax ABH. Biagi et al. compared cerebral perfusion in three age-cohorts (children, teenagers, and adults) with CBF value measured by the arterial spin labeling technique in magnetic resonance imaging [3]. They describe a reduction of CBF especially in the grey matter with increasing age [3]. Most people in the elderly suffer from diseases such as hypertension [15], which leads to confluent white matter lesions [24]. Bastos-Leite et al. showed that these lesions are associated with a reduced brain perfusion [1]. This underlines the importance of considering that perfusion values differ between age groups and that even minor injuries in older people may lead to a worse outcome than more severe injuries in younger patients [16].
We explain the difference of Tmax between ABH and mABH with a nonfunctional autoregulation. Slotty et al. showed that autoregulation functions in chronic SDH probably because the hematoma grows slowly. They found an elevated CBV and CBF in ABH and no difference of Tmax and MTT between ABH and mABH [20]. This leads to the possible explanation that CBV and CBF do not adapt quickly in the case of aSDH because of its sudden growth and therefore Tmax elevates. This could reveal the absence of autoregulation with the consequence of hypoperfused brain tissue. It remains unclear whether the autoregulation defect is based on a higher ICP due to mass effect or on the trauma and following biochemical pathways. Today, therapeutic decisions are based in part on the detection of mass shift on native CT scans. Table 1 shows that patients with a large hematoma thickness or midline shift were more likely to be treated surgically. Salvant et al. mentioned the importance of mass effect and elevated ICP in aSDH. They compared two groups of patients: group one had major lesions with mass effect or midline shift after TBI and group two consisted of TBI patients without mass effect [17]. Group one with mass effect showed comparatively a decreased CBF in the first 48 h of injury. The patients with aSDH often developed secondary brain ischemia, measured with the cerebral metabolic rate of oxygen consumption. As mentioned before, Tmax is a delay-dependent value, so its elevation in ABH could be based on an elevated ICP especially in the affected region. Unfortunately, ICP values were not measured in our study.
The expected elevation of ICP in aSDH was observed in some studies and was often caused by brain swelling [18]. De Souza et al. mentioned that brain swelling results in a disproportion between hematoma thickness and midline shift [6]. This study included 114 patients with aSDH. De Souza et al. analyzed the Zumkeller index (\(ZI=midline\;shift-hematoma\;thickness\)), an indicator of brain swelling, as a prognostic factor and found out that a ZI > 3 is a predictor of mortality in patients with aSDH with odds ratio of 8.12. Nearly half of the patients with a ZI > 3 also had an intraventricular hemorrhage, which is associated with a poor prognosis [6, 13]. This supports the correlation between midline shift and Tmax ABH found in our study.
Limitations
As limitations of the study we identify the retrospective design and the fact that no long-term data are available. Although very difficult in such a study, ICP measurements would have been of great value. Another limitation is that it was not possible to calculate the impact of underlying pathologies like contusion hemorrhage, intracerebral bleedings, etc. on Tmax. For that, we plan to conduct further studies.
Conclusions
Tmax seems to be a relevant marker for the outcome after aSDH, but its therapeutical importance remains unclear. Further prospective studies are needed with a larger and more selective study population. Also, studies which calculate the impact of other intracranial bleedings like contusion hemorrhage or intracerebral bleedings are needed and scheduled. In the future, Tmax may possibly become an influencing factor for surgical management decisions when clinical aspects are not clear.
Data availability
Image data were extracted from the PACS of the Düsseldorf University Hospital. All clinical data were obtained from Medico of the Düsseldorf University Hospital.
Abbreviations
- aSDH:
-
Acute subdural hematoma
- CTP:
-
Computer tomography with perfusion imaging
- GCS:
-
Glasgow Coma scale
- AAH:
-
Area anterior to the hematoma
- ABH:
-
Area below hematoma
- PAH:
-
Area posterior to the hematoma
- mAAH:
-
Mirrored area anterior the hematoma
- mABH:
-
Mirrored area below the hematoma
- mPAH:
-
Mirrored area posterior the hematoma
- CBF:
-
Cerebral blood flow
- CBV:
-
Cerebral blood volume
- MTT:
-
Mean transit time
- Tmax:
-
Time to maximum
- TBI:
-
Traumatic brain injury
- ICP:
-
Intracerebral pressure
References
Bastos-Leite AJ, Kuijer JP, Rombouts SA, Sanz-Arigita E, van Straaten EC, Gouw AA, van der Flier WM, Scheltens P, Barkhof F (2008) Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol 29:1296–1301. https://doi.org/10.3174/ajnr.A1091
Bendinelli C, Bivard A, Nebauer S, Parsons MW, Balogh ZJ (2013) Brain CT perfusion provides additional useful information in severe traumatic brain injury. Injury 44:1208–1212. https://doi.org/10.1016/j.injury.2013.03.039
Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A, Tosetti M (2007) Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging 25:696–702. https://doi.org/10.1002/jmri.20839
Biggs D, Silverman ME, Chen F, Walsh B, Wynne P (2019) How should we treat patients who wake up with a stroke? A review of recent advances in management of acute ischemic stroke. Am J Emerg Med 37:954–959. https://doi.org/10.1016/j.ajem.2019.02.010
Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A (2010) The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke 41:1169–1174. https://doi.org/10.1161/STROKEAHA.110.580670
de Souza MR, Fagundes CF, Solla DJF, da Silva GCL, Barreto RB, Teixeira MJ, Oliveira de Amorim RL, Kolias AG, Godoy D, Paiva WS (2021) Mismatch between midline shift and hematoma thickness as a prognostic factor of mortality in patients sustaining acute subdural hematoma. Trauma Surg Acute Care Open 6:e000707. https://doi.org/10.1136/tsaco-2021-000707
Douglas DB, Chaudhari R, Zhao JM, Gullo J, Kirkland J, Douglas PK, Wolin E, Walroth J, Wintermark M (2018) Perfusion imaging in acute traumatic brain injury. Neuroimaging Clin N Am 28:55–65. https://doi.org/10.1016/j.nic.2017.09.002
Herweh C, Juttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, Sartor K, Schramm P (2007) Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke 38:2941–2947. https://doi.org/10.1161/STROKEAHA.107.486977
Huang AP, Tsai JC, Kuo LT, Lee CW, Lai HS, Tsai LK, Huang SJ, Chen CM, Chen YS, Chuang HY, Wintermark M (2014) Clinical application of perfusion computed tomography in neurosurgery. J Neurosurg 120:473–488. https://doi.org/10.3171/2013.10.jns13103
Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T (2014) Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo) 54:887–894. https://doi.org/10.2176/nmc.cr.2014-0204
Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA, Chang CWJ, Warach S, Fazekas F, Inoue M, Tipirneni A, Hamilton SA, Zaharchuk G, Marks MP, Bammer R, Albers GW (2012) MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. The Lancet Neurology 11:860–867. https://doi.org/10.1016/s1474-4422(12)70203-x
Lee JJ, Segar DJ, Morrison JF, Mangham WM, Lee S, Asaad WF (2018) Subdural hematoma as a major determinant of short-term outcomes in traumatic brain injury. J Neurosurg 128:236–249. https://doi.org/10.3171/2016.5.JNS16255
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW (2005) Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57:1173–1182. https://doi.org/10.1227/01.neu.0000186013.63046.6b. (discussion 1173-1182)
Maegele M, Lefering R, Sakowitz O, Kopp MA, Schwab JM, Steudel WI, Unterberg A, Hoffmann R, Uhl E, Marzi I (2019) The incidence and management of moderate to severe head injury. Dtsch Arztebl Int 116:167–173. https://doi.org/10.3238/arztebl.2019.0167
Mosley WJ 2nd, Lloyd-Jones DM (2009) Epidemiology of hypertension in the elderly. Clin Geriatr Med 25:179–189. https://doi.org/10.1016/j.cger.2009.01.002
Rickels E, von Wild K, Wenzlaff P (2010) Head injury in Germany: a population-based prospective study on epidemiology, causes, treatment and outcome of all degrees of head-injury severity in two distinct areas. Brain Inj 24:1491–1504. https://doi.org/10.3109/02699052.2010.498006
Salvant JB Jr, Muizelaar JP (1993) Changes in cerebral blood flow and metabolism related to the presence of subdural hematoma. Neurosurgery 33:387–393
Sawauchi S, Marmarou A, Beaumont A, Signoretti S, Fukui S (2004) Acute subdural hematoma associated with diffuse brain injury and hypoxemia in the rat: effect of surgical evacuation of the hematoma. J Neurotrauma 21:563–573
Slotty PJ, Kamp MA, Beez T, Beenen H, Steiger HJ, Turowski B, Hanggi D (2015) The influence of decompressive craniectomy for major stroke on early cerebral perfusion. J Neurosurg 123:59–64. https://doi.org/10.3171/2014.12.JNS141250
Slotty PJ, Kamp MA, Steiger SH, Cornelius JF, Macht S, Stummer W, Turowski B (2013) Cerebral perfusion changes in chronic subdural hematoma. J Neurotrauma 30:347–351. https://doi.org/10.1089/neu.2012.2644
Turowski B, Schramm P (2015) An appeal to standardize CT- and MR-perfusion. Clin Neuroradiol 25(Suppl 2):205–210. https://doi.org/10.1007/s00062-015-0444-5
Vilela P, Rowley HA (2017) Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol 96:162–172. https://doi.org/10.1016/j.ejrad.2017.08.014
Wintermark M, Van Melle G, Schnyder P, Revelly J-P, Porchet F, Regli L, Meuli R, Maeder P, Chioléro R (2004) Admission perfusion CT: prognostic value in patients with severe head trauma. Radiology 232:211–220
Zhao Y, Ke Z, He W, Cai Z (2019) Volume of white matter hyperintensities increases with blood pressure in patients with hypertension. J Int Med Res 47:3681–3689. https://doi.org/10.1177/0300060519858023
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
PJS and MAK conceived the idea. JW and GSP developed the project. CR and BBH assisted with the evaluation of CT perfusion data. JW and GSP wrote the manuscript with input from all authors. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of the Medical Faculty of the Heinrich-Heine-University Düsseldorf and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Winkler, J., Piedade, G.S., Rubbert, C. et al. Cerebral perfusion changes in acute subdural hematoma. Acta Neurochir 165, 2381–2387 (2023). https://doi.org/10.1007/s00701-023-05703-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05703-6