Abstract
Objective
Chronic hydrocephalus requiring shunt placement is a common complication of aneurysmal subarachnoid hemorrhage (SAH). Different risk factors and prediction scores for post-SAH shunt dependency have been evaluated so far. We analyzed the value of ventricle measurements for prediction of the need for shunt placement in SAH patients.
Methods
Eligible SAH cases treated between 01/2003 and 06/2016 were included. Initial computed tomography scans were reviewed to measure ventricle indices (bifrontal, bicaudate, Evans’, ventricular, Huckman’s, and third ventricle ratio). Previously introduced CHESS and SDASH scores for shunt dependency were calculated. Receiver operating characteristic analyses were performed for diagnostic accuracy of the ventricle indices and to identify the clinically relevant cut-offs.
Results
Shunt placement followed in 221 (36.5%) of 606 patients. In univariate analyses, all ventricular indices were associated with shunting (all: p<0.0001). The area under the curve (AUC) ranged between 0.622 and 0.662. In multivariate analyses, only Huckman’s index was associated with shunt dependency (cut-off at ≥6.0cm, p<0.0001) independent of the CHESS score as baseline prediction model. A combined score (0–10 points) containing the CHESS score components (0–8 points) and Huckman’s index (+2 points) showed better diagnostic accuracy (AUC=0.751) than the CHESS (AUC=0.713) and SDASH (AUC=0.693) scores and the highest overall model quality (0.71 vs. 0.65 and 0.67), respectively.
Conclusions
Ventricle measurements are feasible for early prediction of shunt placement after SAH. The combined prediction model containing the CHESS score and Huckman’s index showed remarkable diagnostic accuracy regarding identification of SAH individuals requiring shunt placement. External validation of the presented combined CHESS-Huckman score is mandatory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) after intracranial aneurysm rupture is known to have several early and delayed complications strongly impacting the further course and outcome of disease [26]. Post-hemorrhagic hydrocephalus is one of these common SAH complications, with early onset as an acute hydrocephalus affecting up to 97% of individuals [16]. However, not all these patients require a permanent cerebrospinal fluid (CSF) diversion, and the rate of the reported shunt dependencies in SAH series is about a third of the cases [16, 27]. To reduce the weaning time of external ventricular drains (EVD), timely selection of the patients in need of a shunt is of clinical relevance [28]. Identification could help prevent CSF infections [15] and shorten the duration of hospital stay [7].
Several clinical studies have already focused on identifying early predictors of shunt dependency after SAH [14, 16, 27]. In order to increase the diagnostic accuracy of prediction of shunt dependency, several authors also attempted the construction of risk scores for the identification of SAH individuals requiring shunt placement, such as the chronic hydrocephalus ensuing from SAH Score (CHESS) [27] and the shunt dependency in SAH (SDASH) [14] scores. These scores are based on well-known risk factors like poor initial clinical condition, acute hydrocephalus, amount and pattern of intracranial bleeding, aneurysm location, and development of cerebral infarction. However, there is still a non-negligible risk of diagnostic inaccuracy of these scores limiting their implementation in the clinical routine.
Therefore, identifying novel risk factors for chronic hydrocephalus after SAH will aid in further improvement of the predictive power of the risk scores for post-SAH shunt dependency. In this context, the morphology of the ventricles might present a potential clinical value. Ventricular enlargement and other changes in ventricle morphology have been described in several other neurological disorders. In particular, the Evans’ index introduced in 1942 was used to diagnose normal pressure hydrocephalus and until now remains included in the criteria of patients’ selection [37, 48]. Also, the clinical value of the ventricular indices in Morbus Alzheimer [31] and multiple sclerosis [2] was reported. As to acute neurological conditions, ventricular enlargement described for traumatic brain injury (neonatal), intraventricular hemorrhage (IVH) and SAH, has been linked with increased morbidity [5]. Finally, there are only few studies on the association between ventricular measurements and shunt dependency after SAH [41, 51]. However, these studies are based on small SAH cohorts and do not compare the diagnostic value of different ventricular indices.
In light of the above-mentioned evidence on the clinical value of ventricle indices, we aimed to analyze different parameters of ventricular morphology concerning early prediction of chronic hydrocephalus necessitating shunt placement in SAH patients. A special emphasis was put on the evaluation of the additive predictive value of ventricular indices in the context of currently available SAH shunt prediction scores.
Methods
Patient population
In this retrospective study, we included all eligible consecutive SAH patients treated at our institution between January 2003 and June 2016. Patients were adults (>18 years) and had an available pre-treatment computer tomography (CT) scan <48h after ictus, enabling the measurement of the different ventricular parameters and survived long enough to evaluate the need for shunt placement. Patients were excluded from the study if they (a) did not receive aneurysm treatment, were (b) transferred elsewhere or (c) deceased before the end of weaning of CSF diversion, and (d) were admitted >48h after ictus or did not have an available CT scan <48h after ictus. The local ethics committee (Ethik-Kommission, Medizinische Fakultät der Universität Duisburg-Essen, Registration number: 15-6331-BO) approved our study. It was conducted within the clinical trial registered at the German trial registry (DRKS, Unique identifier: RKS00008749).
SAH management
To confirm the rupture of an intracranial aneurysm, all patients underwent digital subtraction angiography (DSA) and were admitted to our neurosurgical intensive care unit. Aneurysms were secured by microsurgical clipping or endovascular coiling, mostly within 24 h after admission. Post-interventional intensive care treatment of SAH patients in our center was already described elsewhere [11, 43] [10, 42]. In short, conservative management consisted of maintenance of normovolemia, mean arterial pressure >70 mmHg, and oral nimodipine for the first 21 days after ictus. Vasospasm surveillance included daily transcranial Doppler ultrasonography with repeated DSA for vasospasm verification and endovascular treatment in SAH patients suspected of symptomatic vasospasm. In case of acute hydrocephalus, an EVD was inserted. If no contraindications such as increased intracranial pressure (ICP) or meningitis were present, patients were weaned from the external CSF drainage after 1 week by closing the EVD for 48 h. Prior to and after disengagement of the EVD, routine CT scans were performed. The weaning was considered successful when patients (a) did not have pathologically increased ICPs (>20 mmHg) in this period; (b) developed no neurological deterioration and/or increased headache reversible by opening of the drain; and (c) showed no increase of ventricle width in the post-challenge CT scan. Additional CT scans were performed with any neurological deterioration, after every surgical intervention, and before and after placement of a ventriculoperitoneal shunt. The indication for shunt placement was based on the presence of above-mentioned clinical and radiographic signs of chronic hydrocephalus and the failure of previous EVD weaning attempts. In SAH patients suspected of chronic hydrocephalus without EVD, a lumbar puncture with measurement of opening pressure was performed.
Data management
For this study, the first available pre-treatment CT scan after admission was reviewed by the first author (M.S.), blinded at this time for any clinical information. The components of the bifrontal, bicaudate, ventricular and third ventricle ratios, and Evans’ and Huckman’s indices were measured, as described in previous literature [3, 17, 20, 22, 32, 39]. A graphic depiction of the different measurements is provided in Fig. 1. Other radiographic parameters assessed with the DSA and CT scans were the location of the ruptured aneurysm, presence of IVH (defined as any evidence of acute intraventricular bleeding visualized in the initial CT scan), the Barrow Neurological Institute (BNI) grading scale [52] at admission, and the development of early cerebral infarcts within 72 h post-SAH according to previous data assessment [29]. To calculate the CHESS and SDASH scores (see supplementary Table S1 in Online Supplements) and record the rates of post-SAH hydrocephalus, initial clinical condition according to the Hunt and Hess scale [24], as well as acute and chronic hydrocephalus requiring shunt placement, was also collected for further analysis. The presence of acute hydrocephalus was judged upon the specific radiographic (ventricle enlargement on the basis of the third ventricle width and periventricular low density on CT scan) and clinical signs (such as mental deterioration, memory impairment, gait disturbance, and urinary incontinence) occurring within 3 days after ictus [1, 14, 27]. To address the possible discrepancy between the true shunt dependency and documented shunt placement rates (due to prophylactic shunt implantation), the whole post-SAH treatment data from the electronic medical records were reviewed with regard to the need for shunt revision or removal surgery due to shunt over-drainage.
Illustration of the ventricular measurements. Bifrontal ratio: maximum width between the two frontal horns (A)/internal width of the vault at same level (a). Bicaudate ratio: minimum width of the ventricles between caudate nuclei (B)/internal width of the vault at same level (b). Ventricular ratio: minimum width of the ventricles (B)/maximum width between frontal horns (A). Third ventricle ratio: greatest width of the third ventricle (C)/internal width of the vault at same level (c). Evans’ index: maximum width between frontal horns (A)/maximum internal width of the vault (D). Huckman’s index: maximum width between the two frontal horns (A) + minimum width of the ventricles between caudate nuclei (B)
Study endpoint and statistical analyses
The purpose of our study was to analyze the additive predictive value of the different ventricular measurements in the context of previously reported shunt dependency risk scores for SAH patients. First, the associations between the ventricular morphology parameters and shunt placement were evaluated in univariate analysis using the Student’s t test for normally distributed continuous data and the Mann–Whitney U test for non-normally distributed continuous data. The significant associations were tested in the receiver operating characteristic (ROC) curves to elucidate the diagnostic accuracy of and identify the clinically relevant cut-off points for all selected ventricular indices. Then, a multivariate backward stepwise regression analysis was performed. As the baseline prediction model, it included the currently available risk score with the highest value of the area under the curve (AUC) in the ROC analysis (the CHESS score). Moreover, the backward stepwise regression analysis included all ventricular parameters as dichotomous variables (according to the ROC-based cut-off values). Finally, the significant results from this multivariate analysis were used for the construction of a novel combined risk score for the prediction of shunt placement after SAH. The weights of the score components were calculated using the values of the adjusted odds ratios (aOR). The significant aORs were divided by the smallest coefficient and rounded to the nearest whole number. After calculating the novel risk score values for all individuals in the cohort, its diagnostic accuracy for the prediction of shunt placement was assessed and compared with the previously introduced risk scores using the ROC analyses: the AUC of the ROC curve, precision–recall curve, and overall model quality.
Data analysis was performed using SPSS statistical software (version 25, SPSS Inc., IBM). Correlations with a p value of ≤0.05 were considered statistically significant.
Results
Of the 995 patients treated at our institution in the mentioned study period, we could include a total of 606 cases in the final cohort after applying the exclusion criteria (see Fig. 2 for the flowchart). An overview of the study-related patients’ characteristics is provided in Table 1.
Two hundred twenty-one individuals (36.5%) in the study cohort received permanent CSF diversion. Of them, five individuals (2.3%) underwent shunt revision surgery (placement of a shunt assistant device) due to shunt over-drainage during the mean post-SAH follow-up time of 41.7 months. Further, there were no cases requiring shunt removal due to over-drainage and missing/resolved chronic hydrocephalus.
In the univariate analysis, we found the values of all ventricular indices significantly higher in the group with shunt (all: p<0.0001) (see Fig. 3). In the subsequent ROC analysis, the values for the AUC for the ventricular parameters ranged from 0.622 to 0.662 (p<0.0001 for all indices) (see supplementary Table S2 in Online Supplements). In comparison, the AUC values for the shunt dependency risk scores were higher, with the CHESS score reaching the best diagnostic performance (AUC=0.713, 95% confidence interval [CI] 0.673–0.754, p<0.0001) followed by the SDASH score (AUC=0.693, 95% CI 0.651–0.734, p<0.0001).
Based on the above-mentioned ROC curves, a clinically relevant cut-off for the prediction of shunt placement was determined for all selected ventricular indices. Thereafter, a multivariate binary logistic backward regression analysis was applied. As the baseline predictor model, the CHESS score was included. In the final stepwise regression, we found only the Huckman’s index (cut-off at ≥ 6 cm, aOR=2.76, 95% CI 1.65–4.62, p<0.0001) to be associated with chronic hydrocephalus independent of the CHESS score (aOR=1.57 per point increase, 95% CI 1.39–1.77, p<0.0001) (see Table 2). Accordingly, both parameters were included in the new risk score for prediction of shunt dependency after SAH containing all original components of the CHESS score (acute hydrocephalus [4 points], Hunt and Hess=4–5 [1 point], IVH [1 point], aneurysm in the posterior circulation [1 point], and early cerebral infarction [1 point]) and the Huckman’s index (≥ 6 cm [2 points]).
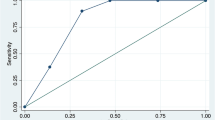
This novel combined CHESS-Huckman score (0–10 points) (see Table 3) was then calculated for the whole cohort and thereafter correlated with the study endpoint. The more points the SAH individuals scored on the CHESS-Huckman scale, the higher the probability of shunt placement in the cohort, ranging from 1.6 to 69% (p<0.0001) (see Fig. 4). The diagnostic accuracy of the combined CHESS-Huckman score was also confirmed in the ROC analysis. The AUC for this novel risk score was 0.751 (95% CI 0.713–0.790, p<0.0001) higher than the AUC for the CHESS and SDASH scores (Fig. 5). The CHESS-Huckman score also performed better than other risk scores in the precision–recall curve. Finally, the Gini index (0.502 vs. 0.427 and 0.385) and the overall model quality (0.71 vs. 0.67 and 0.65) showed the novel combined CHESS-Huckman score’s superiority when compared with the CHESS and SDASH scores, respectively.
When further analyzing the diagnostic performance of the combined CHESS-Huckman score in the cohort, we did not identify significant difference in the score values between shunted SAH individuals who did, or did not, require shunt revision surgery due to over-drainage (median 7.0 vs 7.0, p=0.414). We also analyzed the impact of baseline characteristics and common SAH complications (ICP increase and cerebral vasospasm) on the probability of false-positive and false-negative prediction of shunt dependency using the novel risk score. So, in the sub-cohort of SAH individuals with low combined CHESS-Huckman score values (0–4 points, n=192), 16 individuals (8.3%) underwent shunt placement despite a low-risk profile according to the score. The parameters significantly associated with the false-negative prediction of shunt placement in this “low shunt risk” sub-cohort were the development of acute hydrocephalus (shunt placement in 16.3% vs. 5.6%, p=0.032), intracerebral hemorrhage (15.5% vs. 5.2%, p=0.024), and cerebral vasospasm on DSA (20% vs. 6.2%, p=0.023) (see supplementary Table S3 in Online Supplements). In turn, in the sub-cohort of SAH individuals with high risk for shunt placement according to the combined CHESS-Huckman score (8–10 points, n=110), 37 patients (33.6%) did not develop shunt dependency during the post-SAH course. Here, only the development of cerebral vasospasm during SAH was significantly associated with the probability of false-positive prediction of shunt dependency in this “high shunt risk” SAH sub-population (no shunt in 15.4% vs 39.3% of cases, p=0.032).
Discussion
Measurement of the ventricles in the initial stage of SAH can give significant information on the dependency on permanent CSF diversion in the later course of disease. With the current data, we showed that especially the Huckman’s index combined with the CHESS score gives a solid and accurate prediction model for the need of shunt placement after SAH.
High variability in the size of ventricle system has been reported in healthy individuals [47], and the patients’ age seems to be the major factor contributing to the ventricular morphology [8, 38]. A change in ventricle size is a common condition in different cerebral pathologies. It can manifest both as ventricle narrowing related to brain swelling or mass effect [46] and as ventricular enlargement as a consequence of idiopathic, post-inflammatory, or post-hemorrhagic hydrocephalus [35]. In particular, increase of ventricle size due to hydrocephalus is a common complication after aneurysm rupture necessitating an external CSF diversion in up to 97% of SAH individuals [16].
Despite extensive efforts in both experimental and clinical studies, the exact mechanisms by which acute and chronic hydrocephalus after SAH are caused remain somewhat elusive. It is thought that an inflammatory reaction lies at the base of both types of hydrocephalus, caused by blood outside of the vessels and in the subarachnoid space [6]. This inflammation could eventually lead to fibrosis and cell death, impeding the normal CSF flow and disrupting the blood–brain barrier [4, 6, 49]. Several biological markers have been found in these pathways, as mentioned by Chen et al. [6]. Besides causing a mechanical obstruction, blood clots are thought to play an important role in CSF hypersecretion, contributing to long-term hydrocephalus [30].
Post-hemorrhagic hydrocephalus is a frequent complication of SAH occurring at different stages of the disease [16, 23, 54]. After the acute and subacute phases (up to 14 days), hydrocephalus might persist despite weaning attempts, necessitating a permanent CSF diversion in about a third of SAH individuals [13, 33, 50, 55]. There is a substantial body of clinical evidence on the link between the risk of acute and chronic hydrocephalus. An almost sixfold increase in odds of shunt dependency was found in SAH patients with acute hydrocephalus, as was shown in a recent meta-analysis by Wilson et al [53]. However, for a more precise estimation of the risk of shunt dependency after SAH, knowledge of other relevant risk factors is of eminent importance. Several clinical research attempts have been conducted in this regard so far.
Since SAH is often accompanied by IVH, quantification of this blood inside the ventricles and its subsequent correlation with the risk of post-SAH hydrocephalus has been a topic of study in the last decades. In 1982, Graeb et al. developed a score to measure the amount of blood inside the ventricles and concluded that delayed hydrocephalus was rather an effect of the SAH than of IVH [19]. This score and its modified variant continue to be used as a tool for chronic hydrocephalus in SAH, although not necessarily intended for this population [9, 36]. Other patient and SAH characteristics repeatedly reported as independent predictors of shunt dependency are poor initial clinical condition, nosocomial meningitis, older age, worse mental function status on admission, anterior and middle cerebral artery aneurysms, coil embolization, vasospasm, and cerebral infarction [12, 40, 45, 57].
The risk scores commonly used in clinical practice allow cumulative assessment of the probability of a specific outcome parameter based on the relevant predictors. The earlier published risk scores like the CHESS [27] and SDASH [14] scores were constructed for the prediction of shunt dependency in SAH and have been validated in other SAH cohorts [56]. Recently, Garcia-Armengol et al. compared the Graeb, Hijdra [21], CHESS, and SDASH scores to predict shunt dependency in aneurysmal SAH patients [18]. While no significant differences were found for the SDASH and CHESS scores as well as the Graeb and Modified Graeb scores, the Hijdra score showed a lower predictive value.
Despite the above-mentioned clinical research, early and proper prediction of shunt dependency after SAH remains a topic of concern in clinical practice. The duration of external CSF diversion is an acknowledged risk factor for developing CSF infections [25, 44]. Additionally, ventriculitis is a dreaded complication with high in-hospital mortality and high rate of neurological deficits in the surviving patients [34]. In order to bring back the number of days of EVD usage and thus minimize the risk of meningitis/ventriculitis and their sequelae, it is of utmost importance to predict which patients are most likely to survive without shunt dependency and which will be dependent on permanent CSF diversion. Thus, the timely removal or internalization of the shunt could be planned, hopefully leading to a reduction in infection rates and their consequences. Moreover, timely and robust prediction of shunt dependency can potentially decrease treatment expenses due to shorter hospital stays and lower hospital readmission rates from rehabilitation centers for secondary shunt placement. These circumstances explain the continuing efforts to improve the diagnostic performance of the prediction tools for shunt dependency after SAH.
As we demonstrated with our data in a large, representative cohort, ventricular measurements on the admission CT scans of SAH patients are a reliable tool for early prediction of the need for shunt placement. The Huckman’s index proved to be an independent predictor for chronic hydrocephalus after SAH. After combining the previously described and validated CHESS score with the Huckman’s index, we found the novel combined score to be superior in diagnostic accuracy than the CHESS and SDASH scores for predicting the necessity of permanent CSF diversion in SAH patients.
The limitations of our study are its retrospective and monocentric design. Due to specific selection criteria aiming to rule out confounding effects on the study endpoint, we had to exclude a large number of patients from our final analyses. We believe, however, that despite this fact, our findings are representative of SAH patients as it remains the largest cohort up to date to compare different risk scores designed for SAH to predict shunt placement. As to the newly introduced combined CHESS-Huckman score, external validation is lacking at this point. This aspect is of particular importance, as the prediction scores previously introduced in the literature have still limited clinical utility. It is most likely due to missing or insufficient proof of diagnostic accuracy of these scores in external SAH cohorts. In our internal validation with different prediction models, however, we found it to be a robust and sensitive predictor of shunt placement in SAH patients. Finally, to address the potential selection bias related to the indications for shunt placement, a prospective evaluation of shunt dependency after SAH in the context of a multi-centric randomized trial is essential.
Conclusions
Prediction of the need for permanent CSF diversion due to chronic hydrocephalus after intracranial aneurysm rupture can be accurately done by the known ventricular measurements at admission. Of these, the Huckman’s index has the highest value for predicting post-SAH chronic hydrocephalus. The combined CHESS-Huckman score provides an outstanding diagnostic accurateness for identifying SAH patients in our cohort who require permanent shunt placement. External validation of this novel score is, of course, necessary. We believe, nevertheless, it could provide a helpful tool for adequate and early selection of SAH patients at risk for chronic hydrocephalus.
References
Bae IS, Yi HJ, Choi KS et al (2014) Comparison of incidence and risk factors for shunt-dependent hydrocephalus in aneurysmal subarachnoid hemorrhage patients. J Cerebrovasc Endovasc Neurosurg 16:78–84. https://doi.org/10.7461/jcen.2014.16.2.78
Bermel RA, Bakshi R, Tjoa C et al (2002) Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch Neurol 59:275–280. https://doi.org/10.1001/archneur.59.2.275
Brinkman SD, Sarwar M, Levin HS et al (1981) Quantitative indexes of computed tomography in dementia and normal aging. Radiology 138:89–92. https://doi.org/10.1148/radiology.138.1.7455102
Chen S, Yang Q, Chen G et al (2015) An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res 6:4–8. https://doi.org/10.1007/s12975-014-0384-4
Chen Q, Feng Z, Tan Q et al Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J Neurol Sci 2017, 375:220–230. https://doi.org/10.1016/j.jns.2017.01.072
Chen S, Luo J, Reis C et al (2017) Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis, and treatment. Biomed Res Int 2017:8584753. https://doi.org/10.1155/2017/8584753
Chung DY, Thompson BB, Kumar MA et al (2022) Association of external ventricular drain wean strategy with shunt placement and length of stay in subarachnoid hemorrhage: a prospective multicenter study. Neurocrit Care 36:536–545. https://doi.org/10.1007/s12028-021-01343-9
Cutler NS, Srinivasan S, Aaron BL et al (2020) Normal cerebral ventricular volume growth in childhood. J Neurosurg Pediatr 26:517–524. https://doi.org/10.3171/2020.5.PEDS20178
Czorlich P, Mende KC, Vettorazzi E et al (2015) Validation of the modified Graeb score in aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 157:1867–1872. https://doi.org/10.1007/s00701-015-2583-5
Darkwah Oppong M, Wrede KH, Muller D et al (2021) PaCO2-management in the neuro-critical care of patients with subarachnoid hemorrhage. Sci Rep 11:19191. https://doi.org/10.1038/s41598-021-98462-2
Darkwah Oppong M, Steinwasser L, Riess C et al (2022) Blood pressure and outcome after aneurysmal subarachnoid hemorrhage. Sci Rep 12:8006. https://doi.org/10.1038/s41598-022-11903-4
de Oliveira JG, Beck J, Setzer M et al (2007) Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery 61:924–933. https://doi.org/10.1227/01.neu.0000303188.72425.24
Dehdashti AR, Rilliet B, Rufenacht DA et al (2004) Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg 101:402–407. https://doi.org/10.3171/jns.2004.101.3.0402
Diesing D, Wolf S, Sommerfeld J et al (2018) A novel score to predict shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 128:1273–1279. https://doi.org/10.3171/2016.12.JNS162400
Dos Santos SC, Fortes Lima TT, Lunardi LW et al (2017) External ventricular drain-related infection in spontaneous intracerebral hemorrhage. World Neurosurg 99:580–583. https://doi.org/10.1016/j.wneu.2016.12.071
Erixon HO, Sorteberg A, Sorteberg W et al (2014) Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: results of a single-center clinical trial. Acta Neurochir (Wien) 156:2059–2069. https://doi.org/10.1007/s00701-014-2200-z
Evans WJ (1942) An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatry 47:931–937
Garcia-Armengol R, Puyalto de Pablo P, Misis M et al (2021) Validation of shunt dependency prediction scores after aneurysmal spontaneous subarachnoid hemorrhage. Acta Neurochir (Wien) 163:743–751. https://doi.org/10.1007/s00701-020-04688-w
Graeb DA, Robertson WD, Lapointe JS et al (1982) Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 143:91–96. https://doi.org/10.1148/radiology.143.1.6977795
Hahn FJ and Rim K. Frontal ventricular dimensions on normal computed tomography. AJR Am J Roentgenol 1976; 126: 593-596. 1976/03/01. https://doi.org/10.2214/ajr.126.3.593.
Hijdra A, Brouwers PJ, Vermeulen M et al (1990) Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke 21:1156–1161. https://doi.org/10.1161/01.str.21.8.1156
Huckman MS, Fox J, Topel J (1975) The validity of criteria for the evaluation of cerebral atrophy by computed tomography. Radiology 116:85–92. https://doi.org/10.1148/116.1.85
Hughes JD, Puffer R, Rabinstein AA (2015) Risk factors for hydrocephalus requiring external ventricular drainage in patients with intraventricular hemorrhage. J Neurosurg 123:1439–1446. https://doi.org/10.3171/2015.1.JNS142391
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20. https://doi.org/10.3171/jns.1968.28.1.0014
Hussein K, Rabino G, Feder O et al (2019) Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: prospective observational cohort study. Acta Neurochir (Wien) 161:517–524. https://doi.org/10.1007/s00701-019-03801-y
Jabbarli R, Reinhard M, Niesen WD et al (2015) Predictors and impact of early cerebral infarction after aneurysmal subarachnoid hemorrhage. Eur J Neurol 22:941–947. https://doi.org/10.1111/ene.12686
Jabbarli R, Bohrer AM, Pierscianek D et al (2016) The CHESS score: a simple tool for early prediction of shunt dependency after aneurysmal subarachnoid hemorrhage. Eur J Neurol 23:912–918. https://doi.org/10.1111/ene.12962
Jabbarli R, Pierscianek D, Ro R et al (2018) Gradual external ventricular drainage weaning reduces the risk of shunt dependency after aneurysmal subarachnoid hemorrhage: a pooled analysis. Oper Neurosurg (Hagerstown) 15:498–504. https://doi.org/10.1093/ons/opy009
Jabbarli R, Darkwah Oppong M, Roelz R et al (2020) The PRESSURE score to predict decompressive craniectomy after aneurysmal subarachnoid haemorrhage. Brain Commun 2:fcaa134. https://doi.org/10.1093/braincomms/fcaa134
Kanat A, Turkmenoglu O, Aydin MD et al (2013) Toward changing of the pathophysiologic basis of acute hydrocephalus after subarachnoid hemorrhage: a preliminary experimental study. World Neurosurg 80:390–395. https://doi.org/10.1016/j.wneu.2012.12.020
Karypidou E, Megagiannis P, Papaoikonomou D et al (2019) Callosal angle and Evans index predict beta amyloid and tau protein in patients with dementia. Hell J Nucl Med 22:51–58
LeMay M (1984) Radiologic changes of the aging brain and skull. AJR Am J Roentgenol 143:383–389. https://doi.org/10.2214/ajr.143.2.383
Li H, Pan R, Wang H et al (2013) Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke 44:29–37. https://doi.org/10.1161/STROKEAHA.112.663559
Luque-Paz D, Revest M, Eugene F et al (2021) Ventriculitis: a severe complication of central nervous system infections. Open Forum Infect Dis 8:ofab216. https://doi.org/10.1093/ofid/ofab216
Milan JB, Jensen TSR, Norager N et al (2023) The ASPECT Hydrocephalus System: a non-hierarchical descriptive system for clinical use. Acta Neurochir (Wien) 165:355–365. https://doi.org/10.1007/s00701-022-05412-6
Morgan TC, Dawson J, Spengler D et al (2013) The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke 44:635–641. https://doi.org/10.1161/STROKEAHA.112.670653
Nakajima M, Yamada S, Miyajima M et al (2021) Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir (Tokyo) 61:63–97. https://doi.org/10.2176/nmc.st.2020-0292
Oguro H, Okada K, Yamaguchi S et al (1998) Sex differences in morphology of the brain stem and cerebellum with normal ageing. Neuroradiology 40:788–792. https://doi.org/10.1007/s002340050685
Pelicci LJ, Bedrick AD, Cruse RP et al (1979) Frontal ventricular dimensions of the brain in infants and children. Arch Neurol 36:852–853. https://doi.org/10.1001/archneur.1979.00500490066011
Rincon F, Gordon E, Starke RM et al (2010) Predictors of long-term shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Clinical article. J Neurosurg 113:774–780. https://doi.org/10.3171/2010.2.JNS09376
Rubinos C, Kwon SB, Megjhani M et al (2022) Predicting shunt dependency from the effect of cerebrospinal fluid drainage on ventricular size. Neurocrit Care. https://doi.org/10.1007/s12028-022-01538-8
Said M, Dinger TF, Gumus M et al (2022) Impact of anemia severity on the outcome of an aneurysmal subarachnoid hemorrhage. J Clin Med 11. https://doi.org/10.3390/jcm11216258
Said M, Gumus M, Herten A et al (2021) Subarachnoid hemorrhage early brain edema score (SEBES) as a radiographic marker of clinically relevant intracranial hypertension and unfavorable outcome after subarachnoid hemorrhage. Eur J Neurol 28:4051–4059. https://doi.org/10.1111/ene.15033
Scheithauer S, Burgel U, Ryang YM et al (2009) Prospective surveillance of drain associated meningitis/ventriculitis in a neurosurgery and neurological intensive care unit. J Neurol Neurosurg Psychiatry 80:1381–1385. https://doi.org/10.1136/jnnp.2008.165357
Shigematsu H, Sorimachi T, Osada T et al (2016) Predictors of early vs. late permanent shunt insertion after aneurysmal subarachnoid hemorrhage. Neurol Res 38:600–605. https://doi.org/10.1080/01616412.2016.1199184
Steed TC, Treiber JM, Brandel MG et al (2018) Quantification of glioblastoma mass effect by lateral ventricle displacement. Sci Rep 8:2827. https://doi.org/10.1038/s41598-018-21147-w
Stratchko L, Filatova I, Agarwal A et al (2016) The ventricular system of the brain: anatomy and normal variations. Semin Ultrasound CT MR 37:72–83. https://doi.org/10.1053/j.sult.2016.01.004
Synek V, Reuben JR, Du Boulay GH (1976) Comparing Evans' index and computerized axial tomography in assessing relationship of ventricular size to brain size. Neurology 26:231–233. https://doi.org/10.1212/wnl.26.3.231
Tan Q, Chen Q, Feng Z et al (2017) Cannabinoid receptor 2 activation restricts fibrosis and alleviates hydrocephalus after intraventricular hemorrhage. Brain Res 1654:24–33. https://doi.org/10.1016/j.brainres.2016.10.016
Varelas P, Helms A, Sinson G et al (2006) Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care 4:223–228. https://doi.org/10.1385/NCC:4:3:223
Weigl C, Bruendl E, Schoedel P et al (2020) III. Ventricle diameter increase during ventricular drainage challenge - a predictor of shunt dependency after subarachnoid hemorrhage. J Clin Neurosci 72:198–201. https://doi.org/10.1016/j.jocn.2019.12.011
Wilson DA, Nakaji P, Abla AA et al (2012) A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography: beyond the Fisher scale. Neurosurgery 71:869–875. https://doi.org/10.1227/NEU.0b013e318267360f
Wilson CD, Safavi-Abbasi S, Sun H et al (2017) Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 126:586–595. https://doi.org/10.3171/2015.11.JNS152094
Yamada S, Ishikawa M, Yamamoto K et al (2015) Aneurysm location and clipping versus coiling for development of secondary normal-pressure hydrocephalus after aneurysmal subarachnoid hemorrhage: Japanese Stroke DataBank. J Neurosurg 123:1555–1561. https://doi.org/10.3171/2015.1.JNS142761
Yang TC, Chang CH, Liu YT et al (2013) Predictors of shunt-dependent chronic hydrocephalus after aneurysmal subarachnoid haemorrhage. Eur Neurol 69:296–303. https://doi.org/10.1159/000346119
Yee SV, Ghani AR, Raffiq A (2020) Review of CHESS score in SAH patients in local malaysian population. J Neurosci Rural Pract 11:113–118. https://doi.org/10.1055/s-0039-3402573
Yu H, Zhan R, Wen L et al (2014) The relationship between risk factors and prognostic factors in patients with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Craniofac Surg 25:902–906. https://doi.org/10.1097/SCS.0000000000000561
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support by the Open Acces Publication Fund of the University of Duisburg-Essen.
Author information
Authors and Affiliations
Contributions
Conceptualization: Maryam Said and Ramazan Jabbarli; methodology: Ramazan Jabbarli; data acquisition: Maryam Said, Meltem Gümüs, Jan Rodemerk, Mehdi Chihi, Laurel Rauschenbach, Thiemo Florin Dinger, Marvin Darkwah Oppong, and Ramazan Jabbarli; formal analysis: Ramazan Jabbarli; writing, original draft preparation: Maryam Said; writing, review and editing: Meltem Gümüs, Jan Rodemerk, Mehdi Chihi, Laurel Rauschenbach, Thiemo Florin Dinger, Marvin Darkwah Oppong, Philipp Dammann, Karsten Henning Wrede, Ulrich Sure, and Ramazan Jabbarli; resources: Ramazan Jabbarli; project administration: Maryam Said; supervision: Ramazan Jabbarli.
Corresponding author
Ethics declarations
Consent to participate
All persons or their relatives gave their informed consent within the written treatment contract signed on admission to our institution.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Said, M., Gümüs, M., Rodemerk, J. et al. The value of ventricular measurements in the prediction of shunt dependency after aneurysmal subarachnoid hemorrhage. Acta Neurochir 165, 1545–1555 (2023). https://doi.org/10.1007/s00701-023-05595-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05595-6