Abstract
Purpose
Hydrocephalus requiring permanent CSF shunting after aneurysmal subarachnoid hemorrhage (aSAH) is frequent. It is unknown which type of valve is optimal. This study evaluates if the revision rate of gravitational differential pressure valves (G-DPVs, GAV® system (B Braun)) (G-DPV) is comparable to adjustable pressure valves (Codman Medos Hakim) (APV) in the treatment of post-aSAH hydrocephalus.
Methods
The use of a gravitational differential pressure valve is placed in direct comparison with an adjustable pressure valve system. A retrospective chart review is performed to compare the revision rates for the two valve systems.
Results
Within the registry from Radboud University Medical Center, 641 patients with a SAH could be identified from 1 January 2013 until 1 January 2019, whereas at the Heinrich Heine University, 617 patients were identified, totaling 1258 patients who suffered from aSAH. At Radboud University Medical Center, a gravitational differential pressure valve is used, whereas at the Heinrich Heine University, an adjustable pressure valve system is used. One hundred sixty-six (13%) patients required permanent ventricular peritoneal or atrial shunting. Shunt dysfunction occurred in 36 patients: 13 patients of the 53 (25%) of the gravitational shunt cohort, and in 23 of the 113 (20%) patients with an adjustable shunt (p = 0.54). Revision was performed at a mean time of 3.2 months after implantation with the gravitational system and 8.2 months with the adjustable shunt system. Combined rates of over- and underdrainage leading to revision were 7.5% (4/53) for the gravitational and 3.5% (4/113) for the adjustable valve system (p = 0 .27).
Conclusion
The current study does not show a benefit of a gravitational pressure valve (GAV® system) over an adjustable pressure valve (CODMAN ® HAKIM®) in the treatment of post-aSAH hydrocephalus. The overall need for revision is high and warrants further improvements in care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gravitational valves are developed to reduce the risk of overdrainage and associated complications, such as subdural effusions in the treatment of hydrocephalus. The use of gravitational valves has been proven beneficial in reducing these complications in different causes of hydrocephalus but especially in idiopathic normal pressure hydrocephalus [8]. Hydrocephalus after aneurysmal subarachnoid hemorrhage (aSAH) is a frequent complication of the disease that occurs in up to 30% of the patients. Next to high- and normal-pressure hydrocephalus, also the gradual change from high- to normal-pressure hydrocephalus in a patient may occur during the course of the disease, which may justify the need for adjustable pressure valves [13].
A recent meta-analysis of two studies investigated the use of fixed differential pressure ventriculoperitoneal shunt valves (DPV) versus adjustable pressure valves (APV) for hydrocephalus following aSAH [7, 13, 17]. It showed that the revision rate was lower in the APV group and a cost–benefit analysis also was in favor of the APV [17]. The Medtronic system was used (Delta versus Strata) in both studies. Although the Delta and Strata system are reported to be gravitational systems, there is only a marginal difference between horizontal and vertical position (about 1.5 cm H20) in contrast to a true gravitational system [11].
This study evaluates if the revision rate of gravitational differential pressure valves (G-DPVs, GAV® system (B Braun)) (G-DPV) is comparable to adjustable pressure valves (Codman Medos Hakim) (APV) in the treatment of post-aSAH hydrocephalus.
Methods
The STROBE guidelines were followed for the collection and reporting of data [20]. Medical records were retrospectively analyzed for all consecutively treated aSAH patients of 18 years or older who required ventriculoperitoneal or ventriculo-atrial shunt placement between January 2013 and January 2019 at the Neurosurgery Departments of both Radboud University Medical Center in Nijmegen, the Netherlands, and the Heinrich Heine University Dusseldorf, Germany. The G-DPV from the MIETHKE GAV® (B Braun, Hessen, Germany) was used at Radboud University Medical Center. The APV from CODMAN ® HAKIM® (CMH) (Codman; Johnson & Johnson Co., Raynham, MA) was used at the Heinrich Heine University Dusseldorf.
The primary outcome was shunt dysfunction, warranting revision of the system. Secondary outcomes were the occurrence of clinical and neuroradiological overdrainage, shunt obstruction and location of obstruction if present, infection rates (defined by at least one positive cerebrospinal fluid (CSF) culture at microbiological evaluation), ventriculomegaly slit ventricles, and a need for adjustment in the APV group.
Clinical overdrainage was defined as clinical symptoms (headache, nausea) occurring in the upright position with prompt disappearance in the prone position [6]. Radiological overdrainage was defined as the enlargement of the subarachnoid space over the convexity > 3 mm (hygroma) or a subdural hematoma thicker than 2 mm or slit ventricles together with disproportionally wide cortical sulci.
Baseline characteristics were registered: age, sex, American Society of Anesthesiologists (ASA) score, World Federation of Neurosurgical Societies (WFNS) grade, modified Fisher grade, procedural complications, the presence of slit ventricles, antithrombotic medication, and location of the shunt (frontal, temporal, bilateral shunts). Follow-up time was defined as the time in months from drain implantation to the last clinical or radiological follow-up appointment.
The EVD weaning protocol differed at the two centers. At the Radboudumc, weaning was performed by closing the drain for 24 h. If patient condition remained stable and the pressure was below 20 cmH2O, the EVD was removed. At the Heinrich Heine University Dusseldorf (HH Dusseldorf), the EVD overflow chamber was raised to 20 cmH2O on day 1 and to 25 cmH2O on day 2. If the patient remained clinically stable, a CT scan was performed at the end of day 2. Thereafter, the external drain could be removed.
Statistical analyses were performed with SPSS (Software SPSS — version 22, SPSS Inc., Chicago, IL, USA). Statistical significance was assumed when p < 0.05. Mean and standard deviations were calculated for continuous, normally distributed variables, while median and interquartiles were provided for continuous, non-normally distributed variables. Frequencies were calculated for all categorical data. Univariate analysis was performed to evaluate differences in baseline. Chi-square or Fisher exact test for all categorical data or independent t test was used when appropriate. Normality for continuous data was tested using the Shapiro–Wilk test. To compare two groups if continuous variables were skewed, the Mann–Whitney U test was used. A Kaplan–Meier survival analysis was performed for shunt revision for the two different systems.
Results
From 1 January 2013 until 1 January 2019, a total of 1258 aSAH patients could be identified; 641 were admitted to Radboud University Medical Center, 617 to Heinrich Heine University. Fifty-three of the 641 aSAH patients (8.2%) at Radboud University Medical Center required permanent ventricular peritoneal or atrial shunting, and this group will be referred to as the G-DPV cohort. At the Heinrich Heine University, 113 of the 617 aSAH patients (18.3%) required permanent ventricular peritoneal or atrial shunting, and this group will be referred to as the APV cohort. The overall combined rate of shunted patients was 13%. In nine cases, the type of shunt was not registered. The selection of patients is shown in Fig. 1. Baseline characteristics are provided in Table 1.
The median time from aSAH until shunt placement was 27 (range 9–285) days at the Radboudumc and 18 (range 5–707) days at Heinrich Heine University. Shunt revision was needed in 13 patients in the G-DPV cohort (24.5%), and in 23 patients in the APV cohort (20.4%) (p = 0.54), resulting in a total revision rate of 21.7%. Shunt revision was performed at a mean time of 3.2 and 8.2 months after implantation at the G-DPV and APV, respectively (p = 0.63). The time until revision is graphically depicted in a Kaplan–Meier curve (Fig. 2). Causes of shunt disfunction are provided in Table 2. In the APV cohort, shunt revision was performed by replacing the entire system without investigation of the exact origin of the obstruction in 10 patients. More than one shunt surgery was needed in seven patients for the G-DPV cohort; a revision was needed in three patients, a second revision in one patient, and a third revision in three. Multiple surgeries were performed in seven patients of the APV cohort; a revision was needed in six patients and a second revision was performed in one patient. Combining overdrainage and underdrainage led to a revision rate of 7.5% (4/53) for the G-DPV and 3.5% (4/113) for the APV system (p = 0 0.27). One or more valve adjustments in the APV group were required in 46 patients (27 cases of underdrainage, 15 of overdrainage, and 4 missing values). Radiological abnormalities (hygroma, slit ventricles, and ventriculomegaly) were present in 3.8% (2/53) of the G-DPV cohort and 26.5% (30/113) of the APV cohort (p = 0.001) but did not require surgical revision. No significant difference was observed between the APV and G-DPV group regarding radiological signs of overdrainage.
Discussion
The present study reports the use of a true G-DPV compared to an APV in hydrocephalus after aSAH. Overall revision rates were 24.5% and 20.4% for the G-DPV and APV system, respectively. Combining over- and underdrainage, the revision rate was 7.5% and 3.5% for the G-DPV and APV system, respectively. Although the rate of revision for over- and underdrainage seemed to differ, it did not reach statistical significance. The overall revision rate was not different for either system.
The reason for revision in the G-DPV cohort was mainly (13.2%) due to a defect valve or occlusion. In the APV cohort, it was not investigated in a substantial number of patients (8.8%) due to local practice. Therefore, conclusions on the causes of specific malfunction other than overdrainage or underdrainage cannot be made in this cohort of patients.
Literature reports on shunting after aSAH
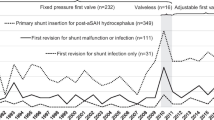
Several studies reported on shunt survival in aSAH using a specified valve type [5, 7, 9, 12, 13] Revision rates ranged from 4.2 to 30.3% (Table 3). Overall revision rate in the extant literature combined with our present results was 18.6%. Three studies reporting the use of the Strata system (APV) had revision rates ranging from 7.0 to 21.0% [7, 9, 13, 18]. Of those, two studies compared the use of the Strata valve to the Delta valve at the same location. Both studies had low revision rates (7.0 and 9.1%). It was at the discretion of the surgeon which valve to use, potentially leading to selection bias. One study had limited follow-up for the APV cohort in comparison with the DPV cohort, which might have influenced the results (5.4 versus 24.9 months) [13]. The results of the published APV revision rates were better compared to the revision rates of the APV cohort in the present study. The only marginal gravitational difference of the Strata system was unlikely to be the explanation of the difference with the CMH valve [11]. One other study reported the use of the CMH valve and had a low revision rate of 4.2%, but also had limited follow-up time [12]. It is known that shunt revisions can occur relatively late, i.e., 1 year after implantation [9]. A prospective randomized trial on the use of the CMH found a revision rate of 52% at 2 years, with a mixed variety of underlying pathology [14].
Additionally, several other studies investigated revision rates of shunts used in the setting of aSAH but did not specify the type of valve used: One report compared pressure-regulated valves (including valveless, non-adjustable pressure valves, and adjustable pressure valves), flow-regulated valves, and shunts with any valve plus a gravitational unit [19]. Revision rates were 25.0% (5/20), 13.3% (2/15), and 33.3% (4/12), respectively; shunt types were not specified and minimum follow-up was 6 months [19]. Another study reported a revision rate of 42.2% (35/83) at the 6-month follow-up, with no specified valve system [16]. In a recently published study, adjustable valve (25/101), valveless (11/16), and fixed pressure valve (75/232) shunts were compared; however, no distinction was made between adjustable valve shunts with (n = 15) and without (n = 86) an anti-siphon component regarding revision rate [18]. Overall revision rates in these non-specific studies were variable but high: 32.8% (157/479).
Shunt dependency after aSAH
The cause of different rates of shunt dependency in the present two cohorts (8.3% and 18.3%) is not clear. Shunt dependency is mostly related to highly modified Fisher grades [21], but these were similar in both groups. Open surgical treatment (a significantly higher percentage in the APV group) is related to a lower non-significant incidence of shunt-dependent hydrocephalus; accordingly, inverse results would be expected [2, 15]. In both centers, the weaning of an external shunt was attempted before deciding for an internal shunt placement, depending on clinical condition in combination with ventriculomegaly or transependymal effusions. Typically, shunt dependency rates after SAH are around 20% [3]. The higher shunt dependency rate could potentially result in a percentage of patients eventually not requiring an internal shunt; a potential dysfunction in these patients would therefore not lead to clinical or radiological abnormalities, lowering the overall risk of revision. Hydrocephalus after SAH is known for its variable occurrence during the early weeks in combination with different pressure characteristics. Commonly, early in the course of the disease, a high-pressure hydrocephalus can occur, whereas a normal-pressure hydrocephalus is seen more often in the later stages. Even changes in the type of hydrocephalus can occur, justifying the need for APV [13]. The presence of acute hydrocephalus requiring shunting within the first 24 h of the disease was not registered in this report; however, baseline characteristics were similar in both groups.
Limitations
This study has several limitations: First, it is limited by its retrospective nature, relying on chart review and available follow-up. For example, in nine patients of the APV cohort, the type of shunt could not be identified and had to be excluded. Despite being a retrospective case series, it is a large consecutive data set in a non-randomized but internally optimized setting. Although a randomized setting might be optimal from a methodological point of view, it is often not optimal for the setting of shunting due to the experience required for a specific type of valve. Pragmatic registry-based observational studies (PROS) might serve as a future methodal framework to answer systematically the question of the optimal valve type [10]. Secondly, follow-up was only performed when clinically indicated or required in the setting of aneurysm control. However, neurological decline would normally lead to hospitalization at the tertiary care hospital, lessening the chance that making it less likely a shunt malfunction would be missed. Differences in indication for shunting leading to differences in rates of shunted patients could also have influenced the results, as discussed above. Thirdly, because a number of neurosurgeons and residents in training performed surgery, differences in expertise levels might have led to differences in revision rates, although ventricular catheter malposition was rare. Last, since each center implanted one type of valve, surgical techniques and local insertion protocols may be a confounder in this study.
Due to the heterogeneity and the nature of the study designs of published literature, no clear conclusion can be drawn on which type of valve is preferable in the setting of hydrocephalus after aSAH. The overall revision rate is high (18.6%) in the series in which the valve type is specified. Associated readmission rates and associated costs are high in patients suffering from hydrocephalus after aSAH [1]. Therefore, future research on improvements in the treatment of post-aSAH hydrocephalus is greatly needed.
Conclusion
The current study does not show a benefit of a gravitational pressure valves (GAV® system) over an adjustable pressure valves (CODMAN ® HAKIM®) in the treatment of post-aSAH hydrocephalus. The overall need for revision is high and warrants further improvements in care.
References
Adil SM, Liu B, Charalambous LT, Kiyani M, Gramer R, Swisher CB, Verbick LZ, McCabe A, Parente BA, Pagadala P, Lad SP (2019) Healthcare economics of hydrocephalus after aneurysmal subarachnoid hemorrhage in the United States. Transl Stroke Res 10:650–663. https://doi.org/10.1007/s12975-019-00697-9
de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, Raabe A (2007) Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery 61:924–933; discussion 933–924. https://doi.org/10.1227/01.neu.0000303188.72425.24
Germanwala AV, Huang J, Tamargo RJ (2010) Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21:263–270. https://doi.org/10.1016/j.nec.2009.10.013
Hayhurst C, Beems T, Jenkinson MD, Byrne P, Clark S, Kandasamy J, Goodden J, Nandoe Tewarie RD, Mallucci CL (2010) Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg 113:1273–1278. https://doi.org/10.3171/2010.3.JNS091237
Hertel F, Zuchner M, Decker C, Schill S, Bosniak I, Bettag M (2008) The Miethke dual switch valve: experience in 169 adult patients with different kinds of hydrocephalus: an open field study. Minim Invasive Neurosurg 51:147–153. https://doi.org/10.1055/s-2008-1065337
Kiefer M, Eymann R, Meier U (2002) Five years experience with gravitational shunts in chronic hydrocephalus of adults. Acta Neurochir (Wien) 144:755–767; discussion 767. https://doi.org/10.1007/s00701-002-0977-7
Lee L, King NK, Kumar D, Ng YP, Rao J, Ng H, Lee KK, Wang E, Ng I (2014) Use of programmable versus nonprogrammable shunts in the management of hydrocephalus secondary to aneurysmal subarachnoid hemorrhage: a retrospective study with cost-benefit analysis. J Neurosurg 121:899–903. https://doi.org/10.3171/2014.3.JNS131088
Lemcke J, Meier U, Muller C, Fritsch MJ, Kehler U, Langer N, Kiefer M, Eymann R, Schuhmann MU, Speil A, Weber F, Remenez V, Rohde V, Ludwig HC, Stengel D (2013) Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA). J Neurol Neurosurg Psychiatry 84:850–857. https://doi.org/10.1136/jnnp-2012-303936
Mansoor N, Solheim O, Fredriksli OA, Gulati S (2021) Revision and complication rates in adult shunt surgery: a single-institution study. Acta Neurochir (Wien) 163:447–454. https://doi.org/10.1007/s00701-020-04526-z
Mansouri A, Cooper B, Shin SM, Kondziolka D (2016) Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered? J Neurosurg 124:558–568. https://doi.org/10.3171/2014.12.JNS142465
Miyake H (2016) Shunt devices for the treatment of adult hydrocephalus: recent progress and characteristics. Neurol Med Chir (Tokyo) 56:274–283. https://doi.org/10.2176/nmc.ra.2015-0282
Nowak S, Mehdorn HM, Stark A (2018) The programmable shunt-system Codman Medos Hakim: a clinical observation study and review of literature. Clin Neurol Neurosurg 173:154–158. https://doi.org/10.1016/j.clineuro.2018.08.023
Orrego-Gonzalez E, Enriquez-Marulanda A, Ascanio LC, Jordan N, Hanafy KA, Moore JM, Ogilvy CS, Thomas AJ (2020) A cohort comparison analysis of fixed pressure ventriculoperitoneal shunt valves with programmable valves for hydrocephalus following nontraumatic subarachnoid hemorrhage. Oper Neurosurg (Hagerstown) 18:374–383. https://doi.org/10.1093/ons/opz195
Pollack IF, Albright AL, Adelson PD (1999) A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery 45:1399–1408; discussion 1408–1311. https://doi.org/10.1097/00006123-199912000-00026
Quigley M (2008) Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery 63:E1209; author reply E1209. https://doi.org/10.1227/01.NEU.0000315869.57200.64
Reddy GK (2012) Ventriculoperitoneal shunt surgery and the incidence of shunt revision in adult patients with hemorrhage-related hydrocephalus. Clin Neurol Neurosurg 114:1211–1216. https://doi.org/10.1016/j.clineuro.2012.02.050
Srivatsan A, Burkhardt JK, Kan P (2020) Commentary: A cohort comparison analysis of fixed pressure ventriculoperitoneal shunt valves with programmable valves for hydrocephalus following nontraumatic subarachnoid hemorrhage. Oper Neurosurg (Hagerstown) 18:E104–E105. https://doi.org/10.1093/ons/opz265
Tervonen J, Adams H, Lindgren A, Elomaa AP, Kamarainen OP, Karkkainen V, von Und Zu, Fraunberg M, Huttunen J, Koivisto T, Jaaskelainen JE, Leinonen V, Huuskonen TJ (2021) Shunt performance in 349 patients with hydrocephalus after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 163:2703–2714. https://doi.org/10.1007/s00701-021-04877-1
von der Brelie C, Meier U, Grawe A, Lemcke J (2016) The dilemma of complicated shunt valves: how to identify patients with posthemorrhagic hydrocephalus after aneurysmatic subarachnoid hemorrhage who will benefit from a simple valve? J Neurosci Rural Pract 7:48–54. https://doi.org/10.4103/0976-3147.172159
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clinical research ed) 335:806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Wilson CD, Safavi-Abbasi S, Sun H, Kalani MY, Zhao YD, Levitt MR, Hanel RA, Sauvageau E, Mapstone TB, Albuquerque FC, McDougall CG, Nakaji P, Spetzler RF (2017) Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 126:586–595. https://doi.org/10.3171/2015.11.JNS152094
Funding
The Department of Neurosurgery of Radboud University Medical Center received an unrestricted grant from Miethke for a hydrocephalus register.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional board review (NL74205.091.20) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Comments
Treatment of hydrocephalus following aneurysmal SAH is complicated, and many factors influence the need for (and the success of) CSF diversion therapies, such as VP shunting. In this retrospective study, Arts et al. investigate whether a gravitational differential pressure valve (GAV® system, B Braun) or a programmable valve (CODMAN® HAKIM®, Integra) is superior regarding need for a subsequent shunt revision. The study was carried out by comparing patient data from a neurosurgical center that used the GAV® system with data from another center that used the CODMAN® HAKIM®. The authors report no statistically significant difference in rate of shunt revision (25% vs 20%) at the two centers (and thus of the two valves). However, the shunt implantation rate differed substantially between the two centers (8% vs 18%). Potentially modifiable and important factors influencing the need of a permanent shunt include timing and strategy (prompt closure vs gradually increasing resistance) of EVD weaning, which unfortunately differed between the two centers being investigated in this study. Although this makes the results difficult to interpret, the study highlights the need for further improvement in shunt treatment to lower the high rate of complications leading to shunt revisions and increased morbidity and mortality in this patient group. The authors should be congratulated for their efforts to investigate the choice of valve as a potential area to advance the care of patients suffering from post-SAH hydrocephalus.
Alexander Lilja-Cyron
Copenhagen, Denmark
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CSF Circulation
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arts, S., van Lieshout, J.H., van Bilsen, M. et al. Non-adjustable gravitational valves or adjustable valves in the treatment of hydrocephalus after aneurysmal subarachnoid hemorrhage patients?. Acta Neurochir 164, 2867–2873 (2022). https://doi.org/10.1007/s00701-022-05361-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05361-0