Abstract
Background
There is increasing evidence that inflammation plays a role in the pathogenesis of aneurysmal subarachnoid hemorrhage (aSAH) and in the development of delayed cerebral ischemia (DCI). However, the assessment and interpretation of classically defined inflammatory parameters is difficult in aSAH patients. The objective of this study was to investigate the relationship between easily assessable findings (hyperventilation, fever, white blood cell count (WBC), and C-reactive protein (CRP)) and the occurrence of DCI and unfavorable neurological outcome at discharge in aSAH patients.
Methods
Retrospective analysis of prospectively collected data from a single center cohort. We evaluated the potential of clinical signs of inflammation (hyperventilation, fever) and simple inflammatory laboratory parameters CRP and WBC to predict unfavorable outcomes at discharge and DCI in a multivariate analysis. A cutoff value for CRP was calculated by Youden’s J statistic. Outcome was measured using the modified Rankin score at discharge, with an unfavorable outcome defined as modified Rankin scale (mRS) > 3.
Results
We included 97 consecutive aSAH patients (63 females, 34 males, mean age 58 years) in the analysis. Twenty-one (22%) had major disability or died by the time of hospital discharge. Among inflammatory parameters, CRP over 100 mg/dl on day 2 was an independent predictor for worse neurological outcome at discharge. The average C-reactive protein level in the first 14 days was higher in patients with a worse neurological outcome (96.6, SD 48.3 vs 56.3 mg/dl, SD 28.6) in the first 14 days after aSAH. C-reactive protein on day 2 was an indicator of worse neurological outcome. No inflammatory parameter was an independent predictor of DCI. After multivariate adjustment, DCI, increased age, and more than 1 day of mechanical ventilation were significant predictors of worse neurological outcome.
Conclusions
Early elevated CRP levels were a significant predictor of worse neurological outcome at hospital discharge and may be a useful marker of later deterioration in aSAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Aneurysmal subarachnoid hemorrhage (aSAH) is a medical emergency associated with substantial long-term morbidity and high mortality [4]. Prediction of functional neurological outcome plays a crucial role in the management of patients affected by this condition. In traditional prediction models, the most common variables associated with an unfavorable neurological outcome are older age, poor neurological status on admission (World Federation of Neurological Surgeons (WFNS) or Hunt and Hess scale), and significant volume of blood on admission CT scan (Fisher grade). However, neurological status is often confounded in unconscious and sedated patients by acute complications such as occlusive hydrocephalus or intracerebral hematoma [1, 3].
Because of emerging evidence of the role of systemic and compartmental inflammation in the pathogenesis of aSAH and in the development of delayed cerebral ischemia (DCI) with or without presence of cerebral vasospasm (CVS), increasing attention is being paid to the relationship between clinical signs of systemic inflammation such as the systemic inflammatory response (SIRS) and neurological outcome in aSAH [5, 16]. In particular, an association between hyperventilation [19, 26, 27], fever [7, 12], and a high white blood cell count (WBC) [18, 20] and unfavorable neurological outcome after aSAH has been described. Moreover, high levels of interleukin 6 (IL-6) and C-reactive protein (CRP) in cerebrospinal fluid (CSF) have been associated with secondary ischemic complications after aSAH, leading to an unfavorable outcome [8, 9, 17]. Serum IL-6 levels have also been associated with DCI and worse outcome [9, 14], although results of studies on serum CRP levels are conflicting [6, 8, 22].
The assessment and interpretation of classically defined and studied parameters of SIRS can be difficult in a clinical setting with severely ill aSAH patients. Catecholamines may be used to induce hypertension to treat CVS-associated DCI; active temperature management may mask fever while the measurement of parameters such as IL-6 may not be widely available, especially in CSF probes, making their use in clinical practice problematic.
The aim of the present study was to investigate the relationship between easily assessable clinical findings (hyperventilation, fever, WBC, and CRP) and the occurrence of DCI, as well as functional neurological outcome in aSAH patients.
Methods
This retrospective analysis of prospectively collected data was performed at a single center. Ethical approval was obtained from the local institutional review board (protocol no. EKSG 12/016). Reporting was done according to the STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology).
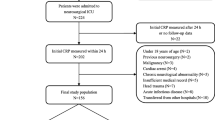
The study included patients aged 18 years and above, who were consecutively admitted to the ICU with a confirmed diagnosis of aSAH between 1 January 2014 and 31 December 2018. All patients with aneurysm occlusion using either endovascular coiling or microsurgical clipping were included. Patients with non-aneurysmatic SAH and patients who did not undergo aneurysm closure were excluded from the analysis.
The clinical severity at the time of admission was assessed by the neurosurgeon on duty according to the WFNS scale and divided into two groups (low grade (WFNS 1–3) and high grade (WFNS 4–5)). Blood volume on the initial computed tomography (CT) scan was assessed by Fisher grade and also categorized into two groups (Fisher 1–2 and 3–4). The modified Rankin scale (mRS) was used to evaluate neurological outcome at hospital discharge.
ICU management of SAH and definitions
All aSAH patients were treated according to the local SAH management guidelines consistent with the Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage by the American Heart Association/American Stroke Association [4].
In addition to general intensive care, all patients were monitored for clinical deterioration. Transcranial Doppler sonography to measure cerebral blood flow velocity in the basal cerebral arteries as a predictor of CVS was performed daily. In cases of new onset neurological deficits or a significant increase in Doppler velocity, CT angiography (CTA) was performed to confirm or exclude the presence of arterial vasospasm. On day 7, a screening CTA was performed in all patients. Fluid management was targeted to maintain euvolemia. Hypertension was induced in cases of delayed neurological deterioration, defined as new onset neurological deficit or a reduction in the Glasgow coma scale of more than 2 points persisting for more than 1 h [19, 20], with a target mean arterial pressure (MAP) of > 110 mmHg. Mannitol or 5% hypertonic saline solution was only administered in cases of elevated intracranial pressure (ICP > 20 mmHg) as a rescue strategy. Interventional single spasmolysis with papaverine with or without angioplasty was reserved for patients who did not respond to induced hypertension. Patients received minimal amounts of sedatives, such as propofol, midazolam, and dexmedetomidine, which were necessary to prevent ventilator dyssynchrony and patient discomfort. Analgesics, including acetaminophen, nonsteroidal anti-inflammatory medications, and fentanyl, were administered according to sedation and pain scores (Richmond agitation sedation scale and behavior pain scale). Fever, defined as body core temperature > 38.2 °C, was treated with acetaminophen, nonsteroidal anti-inflammatory medications, or cooling devices in ventilated patients. Active maintenance of normothermia with cooling devices was not routinely performed.
Ventilation
All patients were intubated with a muscle relaxant prior to angiography and initial treatment (surgical clipping or endovascular coiling). The muscle relaxant was discontinued after completion of the initial treatment and was not used as ICU management thereafter. For mechanically ventilated patients, respiratory management involved the use of the spontaneous breathing mode (pressure support mode) with minimal support (i.e., continuous positive airway pressure or a pressure support level ≤ 5 cm H2O). Daily spontaneous breathing trials were not performed. When a patient’s condition stabilized, the patient was weaned off mechanical ventilation and extubated after the completion of the initial treatment.
Delayed cerebral ischemia
Delayed cerebral ischemia was defined according to the 2010 Multidisciplinary Research Group Definition as the occurrence of secondary neurological deficits or strokes not attributable to another cause. Awake patients with a clinical diagnosis of DCI or patients who required sedation but had suggestive changes in extended neuromonitoring (near-infrared spectroscopy, brain tissue oxygen tension, and cerebral microdialysis) were subjected to radiographic verification. Based on CT angiography and CT perfusion, rescue therapy was initiated [23, 24].
PaCO2
Arterial blood gas (ABG) analyses, including PaCO2, were routinely performed at least every 12 h for the first 14 days. The lowest PaCO2 value was determined for each 24-h period.
Body core temperature
Body core temperature was measured continuously using a bladder catheter with a temperature probe. All patients with fever (> 38.2 °C) underwent an infection workup including urine, sputum, blood culture and, in patients with an external ventricular drain, additional cerebrospinal fluid workup. We defined fever days those on which the body temperature could not be reduced below 38.2 °C by fever management.
Data sampling
The following data were collected from medical charts and the patient data management system: sex, age, Fisher grade, WFNS grade, treatment modality (coiling or clipping), angiographic CVS, occurrence of DCI, ventilatory support (mechanical ventilation, NIV), PaCO2 measurements (days < 4.5 kPa, CO2 mean, day 0–14), body core temperature in °C (Tmean, days > 37.5, days > 38.2, day 0–14), leucocyte count (mean and maximal), CRP level (mean and maximal), functional neurological outcome at hospital discharge (mRS), and hospital mortality. As part of standard treatment, CRP and WBC were measured daily until discharge or at least until day 14. This was not done in less than 10% of cases. The incidence of pneumonia, ventriculitis, and the overall rate of infection were also reported.
Outcome measures
The primary outcome of our study was the ability of clinical signs of inflammation (hyperventilation, fever) and simple inflammatory laboratory parameters (CRP and WBC) to predict unfavorable outcome at discharge defined mRS > 3. The secondary outcome was the ability of the same variables to predict DCI.
Statistical analysis
The statistical analyses were performed using the R statistical software version 4.0.2. (www.r-project.org). A two-sided p value < 0.05 was considered statistically significant.
To assess the diagnostic value of continuous predictors for a dichotomized mRS (mRS 4–6 versus mRS 0–3), receiver operating characteristic (ROC) curve analysis was performed using the R library “PRROC.” Optimal cutoff values were determined using the Youden’s J statistic method. The time course of continuous predictors was assessed by trajectories. To assess the neurological outcome measured by mRS, univariable and multivariable cumulative linkage models were applied using the R library “ordinal.” The multivariable model was complemented by a backward variable selection based on the Akaike’s information criterion (AIC). The predictors were selected according to the literature and considering the univariable results of the ROC analysis and trajectories. They were CVS, DCI, Fisher grade, WFNS scale, age, sex, coiling versus clipping, CRP on day 2 (dichotomized lower vs higher than 100 mg/dl), invasive ventilation longer than 1 day, number of days with fever (T > 38.2 °C), and hyperventilation (lowest daily PaCO2 < 4.5 kPa). C-reactive protein at day 2 was chosen because the extent of inflammation at this stage is less likely to be influenced by ICU-acquired infections and may be more representative of the burden of early brain injury caused by the aSAH itself.
To avoid separation, mRS grades 5 and 6, Fisher grades 1 and 2, and WFNS were included with aggregated categories. Because of strong collinearity with CRP, WBC was not included in this model. To further assess the diagnostic value of the predictors for the severity of the neurologic impairment measured by the mRS in the multivariable context and taking into account the ordinal scale of mRS, a random forest analysis was performed using the “cforest” function from the R library “party” to compute the relative variable importance for the prediction [21].
Univariable and multivariable adjusted logistic regressions were performed for the analysis of the secondary outcomes DCI, supplemented by backward variable selection based on AIC. Missing data were corrected using the random forest method.
Results
Baseline characteristics of patients
A total of 97 consecutive patients with aSAH admitted to the ICU during the study period were consecutively included in the analysis. Of these, 76 (78%) had a favorable neurological outcome at discharge (mRS 0–3) while 21 (22%) had major disability or died (mRS 4–6). Sixty-three patients (64.9%) were female and 34 (35.1%) were male with a mean age of 58 (SD 13.8) years. Demographic characteristics and treatment modality did not differ significantly between patients with favorable and unfavorable neurological outcome, although patients with unfavorable neurological outcome (mRS 4–6) had more severe clinical and radiological presentation at admission (Table 1). Regarding the secondary outcome, 20 patients (21%) had DCI (Table 2).
Primary outcome: prediction of unfavorable neurological outcome (mRS 4–6)
In univariable analysis, ischemic complications (OR 3.18, CI 1.37–7.58 for CVS and 10.23, CI 3.42–31.88 for DCI), higher Fisher grade (OR 1.48, CI 0.53–4.20 for grade III and 4.08, CI 1.57–11.0 for grade IV), WFNS grades 4–5 (OR 4.37, CI 2.07–9.52), CRP on the second day after diagnosis (OR 7.14, 3.09–17.27), more than 1 day of invasive mechanical ventilation (OR 11.22, CI 4.95–26.85), and number of days with hyperventilation (OR 1.24 per day, CI 1.10–1.42) and with fever (OR 1.24, CI 1.10–1.40 per day) were associated with worse neurological outcome at discharge as defined by mRS. Sex and treatment modality did not show significant difference in outcome. A tendency for worse outcome with increased age (OR 1.02, CI 1.00–1.05) was observed (Table 3). After multivariate adjustment, ischemic complications (OR 4.49, CI 1.31–15.93 for CVS and OR 18.04, CI 3.68–93.44 for DCI), increased age (OR 1.05, CI 1.01–1.08), and more than 1 day of mechanical ventilation (OR 5.45, CI 1.76–17.40) were significant predictors for a worse neurological outcome. In the model established by a backwards variable selection procedure, a CRP over 100 mg/dl on day 2 after diagnosis (OR 2.70, CI 1.06–6.98) was another independent predictor for worse outcome (Table 3).
Mean CRP values were consistently higher among patients with worse neurological outcome (96.6, SD 48.3 vs 56.3 mg/dl, SD 28.6, p < 0.001) for the first 14 days after aSAH (Fig. 1). When dichotomizing the outcome (mRS 0 to 3 versus 4 to 6), receiver operating characteristic (ROC) curve analysis revealed the highest diagnostic value for CRP on day 2 (AUC 0.79, CI 0.67–0.91) and days with mechanical ventilation (AUC of 0.87, CI 0.80–0.94) (Figs. 2 and 3). Mean WBC was also higher in patients with mRS 4–6 at discharge (12.0, SD 2.3 vs 10.6 G/l, SD 2.2, p = 0.019), while there was no statistically significant difference in mean PaCO2 (4.5, SD 0.5 vs 4.5, SD 0.5 kPa, p = 0.054) and mean body temperature (37.8, SD 0.5 vs 37.6 °C, SD 0.4, p = 0.329) between the groups. The incidence of pneumonia was higher in patients with worse neurological outcome (6.6% vs 23.8%, p = 0.042), while the overall occurrence of infection barely reached statistical significance (14.5% vs 33.3%, p = 0.069). However, no infection was observed before day 3 after aSAH (Table 2).
Secondary outcome: prediction of delayed cerebral ischemia
In univariable and multivariable analysis, the only predictor for DCI was the number of days of hyperventilation (OR 1.32, CI 1.02–1.87) (Table 4).
Discussion
In our study, early elevated CRP levels along with ischemic complications such as DCI, increased age, and more than 1 day of mechanical ventilation were significant predictors of worse neurological outcome at hospital discharge. Despite a significant improvement in outcomes, aSAH still bears high levels of morbidity and mortality [13, 25]. Easily obtainable early predictors of clinical deterioration can help guide therapy, monitoring, and diagnostic procedures in these patients. In our analysis, a serum CRP level greater than 100 mg/l on the second day of hospitalization was the inflammatory marker with the strongest independent correlation with a worse neurological outcome (Fig. 4). Serum CRP on day 2 also had the highest diagnostic discriminatory power for worse neurological outcome in the ROC curve analysis AUC of 0.79. Fever and hyperventilation were also associated with a worse neurological outcome, but were not predictive of higher mRS after multivariate adjustment. Moreover, mean pCO2 and temperature did not differ between the dichotomized mRS groups. However, consistent with previous studies the number of days of hyperventilation was an independent predictor of DCI [19, 26, 27]. As expected, duration of mechanical ventilation was also associated with worse neurological outcome as it represents a surrogate of disease severity (Fig. 3).
Our findings are consistent with previous reports of Jeon et al., who described an association between CRP on the first 2 days after securing of the aneurysm and the occurrence of unfavorable outcome, while using a lower cutoff of 40 mg/l [10]. Similar findings have also been described in a post hoc analysis of the STASH trial, where CRP was an early independent predictor of poor outcome, even in patients with good radiological and clinical grading at presentation [22]. Other studies found an association between elevated CRP in the early phase after aSAH and higher disability scores but failed to show that it was an independent predictor of worse outcome [8, 11, 15]. Muroi et al. also found an association between elevated CRP and unfavorable neurological outcome and observed higher CRP levels in these patients, especially between days 8 and 14 after admission. However, in contrast to our results, they found that CRP was not an independent predictor of neurological outcome after correction for concomitant infection [14]. A possible explanation for this difference is the overall much higher CRP level in our patient cohort, especially in the first week after admission, which may reflect later hospital presentation. Our findings support the hypothesis that early systemic inflammation may play an important role in the pathogenesis of brain injury in aSAH. Interestingly found no significant association between CRP levels and DCI. We believe that this discrepancy may reflect other mechanisms of early brain injury that play a role in the early phase after aSAH such as transient global ischemia and intracranial pressure elevation after aneurysmal rupture, neuroinflammation, dysfunction of the blood–brain barrier as well as cortical spreading depolarizations [2]. We acknowledge that, because of its lack of specificity, serum CRP may be influenced by infection and sepsis in an ICU population. However, in our analysis the best predictor of neurological outcome among inflammatory markers and signs was the CRP level on the second day of hospitalization which is unlikely to be influenced by infections because these classically occur later in the course of the disease. Furthermore, no episode of infection, including pneumonia, was diagnosed or treated before day 3 in our patient population. We believe that our results provide further evidence that CRP may serve as an early predictor of clinical deterioration, independent of the initial radiologic and clinical grading of aSAH and the occurrence of ischemic complications. Further research is needed to determine whether patients with higher CRP levels might benefit from more intensive monitoring for deterioration and whether a correlation between systemic inflammation and aSAH morbidity exists.
Limitations
Some data, such as body temperature, may have been influenced by the use of analgesics with antipyretic effects and targeted temperature management, while pCO2 levels may have been confounded by controlled mechanical ventilation, as patients with a higher modified Rankin score had more days of mandatory mechanical ventilation (5.1 vs 1.7), making spontaneous hyperventilation impossible. Neurological outcome at discharge may represent a relative short follow-up time. In addition, the CRP cutoff of 100 mg/dl was retrospectively determined by optimizing the Youden’s analysis of ROC data and the choice of day 2 was arbitrary to minimize the influence of ICU-acquired infections. Another limitation of our study is the absence of precise data reporting the frequency and technique of salvage spasmolysis in patients’ refractory to induced hypertension. An additional limitation is the absence of modified Fisher scale data in our analyses; this could have been a potentially better association on the risk of DCI. Finally, we found inconsistent signals concerning the association of infection with DCI and outcome; however, the analysis of this effect was not the objective of our analysis and should be investigated in a larger cohort.
Conclusion
Early elevated CRP levels and ischemic complications such as DCI (OR 3.54 for CVS and 12.10 for DCI), older age, and more than 1 day of mechanical ventilation were significant predictors for a worse functional neurological outcome at hospital discharge. Clinicians should be aware that a high CRP level may be a marker of subsequent deterioration even in patients presenting with good grade aSAH.
References
Al-Khindi T, Macdonald RL, Schweizer TA (2010) Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 41:e519-536. https://doi.org/10.1161/STROKEAHA.110.581975
Amodio S, Bouzat P, Robba C, Taccone FS (2020) Rethinking brain injury after subarachnoid hemorrhage. Crit Care 24:612. https://doi.org/10.1186/s13054-020-03342-2
van Donkelaar CE, Bakker NA, Birks J, Veeger NJGM, Metzemaekers JDM, Molyneux AJ, Groen RJM, van Dijk JMC (2019) Prediction of outcome after aneurysmal subarachnoid hemorrhage. Stroke 50:837–844. https://doi.org/10.1161/STROKEAHA.118.023902
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke C, Council on Cardiovascular R, Intervention, Council on Cardiovascular N, Council on Cardiovascular S, Anesthesia, Council on Clinical C (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Dhar R, Diringer MN (2008) The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care 8:404–412. https://doi.org/10.1007/s12028-008-9054-2
Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, Fritzinger M, Horn P, Vajkoczy P, Kreisel S, Brunner J, Schmiedek P, Hennerici M (2001) Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry 70:534–537. https://doi.org/10.1136/jnnp.70.4.534
Fernandez A, Schmidt JM, Claassen J, Pavlicova M, Huddleston D, Kreiter KT, Ostapkovich ND, Kowalski RG, Parra A, Connolly ES, Mayer SA (2007) Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology 68:1013–1019. https://doi.org/10.1212/01.wnl.0000258543.45879.f5
Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP, Robinson JS Jr (2009) Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus 26:E22. https://doi.org/10.3171/2009.2.FOCUS08311
Graetz D, Nagel A, Schlenk F, Sakowitz O, Vajkoczy P, Sarrafzadeh A (2010) High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage. Neurol Res 32:728–735. https://doi.org/10.1179/016164109X12464612122650
Jeon YT, Lee JH, Lee H, Lee HK, Hwang JW, Lim YJ, Park HP (2012) The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 24:317–324. https://doi.org/10.1097/ANA.0b013e31826047a2
Juvela S, Kuhmonen J, Siironen J (2012) C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir 154:397–404. https://doi.org/10.1007/s00701-011-1243-7
Lai PMR, See AP, Silva MA, Gormley WB, Frerichs KU, Aziz-Sultan MA, Du R (2019) Noninfectious fever in aneurysmal subarachnoid hemorrhage: association with cerebral vasospasm and clinical outcome. World Neurosurg 122:e1014–e1019. https://doi.org/10.1016/j.wneu.2018.10.203
Lovelock CE, Rinkel GJ, Rothwell PM (2010) Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 74:1494–1501. https://doi.org/10.1212/WNL.0b013e3181dd42b3
Muroi C, Hugelshofer M, Seule M, Tastan I, Fujioka M, Mishima K, Keller E (2013) Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery 72:367–375; discussion 375. https://doi.org/10.1227/NEU.0b013e31828048ce
Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A (2006) Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 18:68–72. https://doi.org/10.1097/01.ana.0000181693.30750.af
Saand AR, Yu F, Chen J, Chou SH (2019) Systemic inflammation in hemorrhagic strokes - a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab 39:959–988. https://doi.org/10.1177/0271678X19841443
Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P (2010) Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care 13:339–346. https://doi.org/10.1007/s12028-010-9432-4
Oh SY, Kwon JT, Hong HJ, Kim YB, Suk JS (2007) Relationship between leukocytosis and vasospasms following aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 41:153–156. https://doi.org/10.3340/jkns.2007.41.3.153
Solaiman O, Singh JM (2013) Hypocapnia in aneurysmal subarachnoid hemorrhage: incidence and association with poor clinical outcomes. J Neurosurg Anesthesiol 25:254–261. https://doi.org/10.1097/ANA.0b013e3182806465
Spallone A, Acqui M, Pastore FS, Guidetti B (1987) Relationship between leukocytosis and ischemic complications following aneurysmal subarachnoid hemorrhage. Surg Neurol 27:253–258. https://doi.org/10.1016/0090-3019(87)90038-3
Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A (2008) Conditional variable importance for random forests. BMC Bioinformatics 9:307. https://doi.org/10.1186/1471-2105-9-307
Turner CL, Budohoski K, Smith C, Hutchinson PJ, Kirkpatrick PJ, Murray GD, collaborators S, (2015) Elevated baseline C-reactive protein as a predictor of outcome after aneurysmal subarachnoid hemorrhage: data from the Simvastatin in Aneurysmal Subarachnoid Hemorrhage (STASH) trial. Neurosurgery 77:786–792; discussion 792-783. https://doi.org/10.1227/NEU.0000000000000963
Vergouwen MD, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care 15:308–311. https://doi.org/10.1007/s12028-011-9586-8
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Wartenberg KE, Mayer SA (2006) Medical complications after subarachnoid hemorrhage: new strategies for prevention and management. Curr Opin Crit Care 12:78–84. https://doi.org/10.1097/01.ccx.0000216571.80944.65
Williamson CASK, Tipirneni R, Roark CD, Pandey AS, Thompson BG, Rajajee V (2015) The association between spontaneous hyperventilation, delayed cerebral ischemia, and poor neurological outcome in patients with subarachnoid hemorrhage. Neurocrit Care 23:330–338. https://doi.org/10.1007/s12028-015-0138-5
Yokoyama S, Hifumi T, Okazaki T, Noma T, Kawakita K, Tamiya T, Minamino T, Kuroda Y (2018) Association of abnormal carbon dioxide levels with poor neurological outcomes in aneurysmal subarachnoid hemorrhage: a retrospective observational study. J Intensive Care 6:83. https://doi.org/10.1186/s40560-018-0353-1
Acknowledgements
We acknowledge Ms. S. Bölli-Tomic, who served as an English language editor for this manuscript.
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception and design of the study. Material preparation, data collection, and analysis were performed by Alessandro Ostini and Urs Pietsch. Rene Warschkow performed the statistical analysis. The first draft of the manuscript was written by Alessandro Ostini and Urs Pietsch. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics review board (protocol no. EKSG 12/016) and was performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments. All patients gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical intensive care
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alessandro, O., Rene, W., Stefan, W. et al. C-reactive protein elevation predicts in-hospital deterioration after aneurysmal subarachnoid hemorrhage: a retrospective observational study. Acta Neurochir 164, 1805–1814 (2022). https://doi.org/10.1007/s00701-022-05256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05256-0